eISSN: 2379-6367


Review Article Volume 10 Issue 2
1Department of Pharmacology, Cerrahpasa Faculty of Medicine, Istanbul University, Turkey
2Department of Medical Pharmacology, Faculty of Medicine, Beykent University, Turkey
3Department of Internal Medicine, Okmeydani Training and Research Hospital, Turkey
4Department of Pharmaceutical Chemistry, School of Pharmacy, Istanbul Medipol University, Turkey
5Department of Pharmacology, Faculty of Pharmacy, Hacettepe University, Sihhiye, Ankara Turkey
Correspondence: Gokhan Faikoglu, Department of Medical Pharmacology, Faculty of Medicine, Beykent University, Turkey
Received: March 24, 2022 | Published: April 18, 2022
Citation: Saygisever-Faikoglu K, Faikoglu G, Ozcan FO, et al. Efficacy and safety of propiverine in patients with overactive bladder and neurogenic detrusor overactivity. Pharm Pharmacol Int J. 2022;10(2):51-58. DOI: 10.15406/ppij.2022.10.00364
Purpose: The purpose of this review is to evaluate the efficacy and safety of propiverine in pediatric and adult patients, which is used in the symptomatic treatment of urgency urinary incontinence and urinary frequency and/or urinary incontinence in patients with neurogenic detrusor overactivity due to overactive bladder or spinal cord injuries.
Method: Literature was retrieved by a PubMed search, using different combinations of pertinent keywords (e.g., propiverine, overactive bladder, neurogenic detrusor overactivity), without any limitations in terms of the publication date and language. Papers that assessed the therapeutic efficacy and tolerability of propiverine in patients with overactive bladder or neurogenic detrusor overactivity were selected for inclusion according to their relevance for the topic, as judged by the authors.
Results: Propiverine is reported to be a significantly effective and safe treatment option in adult and pediatric patients with overactive bladder or neurogenic detrusor overactivity. It is observed that propiverine provides an increase in life quality without affecting cognitive functions, and may be a safe treatment option, except for patients with angle-closure glaucoma, who have not been examined in terms of ophthalmological reliability. It was observed that no changes were observed in cardiac parameters in patients receiving propiverine treatment, and there was no clinically significant difference in the incidence of side effects between the two groups in studies comparing elderly and young patients. Propiverine is recommended by the pediatric committee at the International Incontinence Consultation with the highest level of evidence (1B/C) of all anticholinergics.
Conclusion: Propiverine is an effective and safe treatment option that can be the first choice in the symptomatic treatment of both adult and pediatric patients with overactive bladder or neurogenic detrusor overactivity, with its high efficacy and significantly higher tolerability.
Keywords: propiverine, overactive bladder, neurogenic detrusor overactivity, antimuscarinics
ICS, International Continence Society; OAB, overactive bladder; UTI, urinary tract infection; ER, extended-release; IR, immediate-release; pKi, binding affinity; ATP, adenosine triphosphate; Ca, calcium; NICE, The National Institute for Health and Care Excellence; CIBIC, Clinician's Interview-Based Impression of Change; PVR, post-void residual; NDO, neurogenic detrusor overactivity; RV, reflex volume; MDP, maximum detrusor pressure; BC, bladder compliance; UI, urge incontinence; Pdetmax, maximum detrusor pressure; IPSS, international prostate symptom score; MCC, maximum cystometric capacity; ECG, electrocardiography
The term "overactive bladder" was first defined in the report of the International Continence Society (ICS) in 1988 as a chronic condition characterized by involuntary bladder contractions during the filling phase of the micturition cycle, defined urodynamically as "detrusor overactivity".1 The definition of OAB was developed by the ICS to include urinary urgency, usually with urinary frequency (more than eight micturitions/24 hours) and nocturia, with or without urgency urinary incontinence (Table 1).2
Symptom |
Definition |
Urge |
Sudden and compelling urge to urinate that is difficult to postpone |
Urgency urinary Incontinence |
Involuntary urinary incontinence accompanied by urgency or occurring immediately after urgency |
Increased frequency of daytime urination |
Frequent urination during the day reported by patients |
Nocturia |
The condition of causing an individual to wake up one or more times during the night to urinate |
Table 1 Definitions of overactive bladder symptoms (Adapted from Abrams P et al.3)
For the diagnosis to be OAB, symptoms must occur in the absence of explanatory pathological (eg, urinary tract infection, bladder stones, or interstitial cystitis) or metabolic factors (eg, diabetes mellitus). Although symptoms suggest detrusor overactivity (urodynamically significant, involuntary bladder contractions), they may also occur due to other storage or excretory dysfunctions. Although OAB is therefore clearly distinguished from urodynamically proven detrusor overactivity, most people with OAB are thought to have this diagnosis as the underlying disease.4 In summary, an overactive bladder is defined as a condition in which urgency urinary incontinence and/or nocturia may accompany in the absence of urinary tract infection (UTI) or other obvious pathology.5
Overactive bladder symptoms can also cause psychological distress and have profound negative effects on quality of life, relationships, and self-esteem.6 International clinical practice guidelines recommend patient education, lifestyle changes, and bladder education for the initial management of overactive bladder or urinary incontinence.7 Overactive bladder is a common chronic disease observed equally in men and women, affecting approximately 17% of the adult population, and the lives of millions of people of all ages worldwide, and. However, its prevalence is higher in the elderly (31% in women aged >75 years, 42% in men).5 It affects 11.8% of adults across all age groups, and its prevalence rises to 19.1% in men aged 60 and over and 18.3% in women aged 60 and over.8
Antimuscarinics are the pharmacological treatment of choice for overactive bladder. Seven antimuscarinics (propiverine, darifenacin, fesoterodine, oxybutynin, solifenacin, tolterodine, and trospium chloride) with different dosages, formulations, and uses of administration are currently used in the treatment of overactive bladder.9 Drugs such as propiverine, oxybutynin, tolterodine, and trospium are available in both immediate-release (IR) and extended-release (ER) formulations, while oxybutynin is also available in a sustained-release patch form for transdermal administration.10
In addition, propiverine has been shown to have a lower affinity for M2 receptors, which are effective in cardiac functions, compared to tolterodine, oxybutynin, darifenacin, and trospium (Table 2).13
Binding affinity (pKi) of antimuscarinic compounds for the human recombinant receptor subtype M2 |
|
Propiverine |
5.4 |
Tolterodine |
8 |
Oxybutynin |
7.8 |
Darifenacin |
7.4 |
Trospium |
9.2 |
Table 2 Binding affinity (pKi) of antimuscarinic compounds for the human recombinant receptor subtype M2 (Adapted from Paul Abrams et al.13)
In addition to its antimuscarinic effect, propiverine can also suppress bladder overactivity by reversing adenosine triphosphate (ATP)-induced overactivity. In rats, bladder overactivity induced by intravesical ATP has been reported to be suppressed by propiverine and oxybutynin.14 Propiverine's dual mechanism of action is a unique antimuscarinic that also exhibits pharmacodynamic effects through both antimuscarinic and musculotropic pathways. Propiverine provides a spasmolytic effect (musculotropic effect) by inhibiting the uptake of calcium in the smooth muscles of the bladder. It also inhibits the efferent connection of the pelvic nerve by muscarinic receptor blockade (anticholinergic effect) and inhibition of ATP-related bladder overactivity. (neurotropic effect).12,21
All of the current antimuscarinics used in the treatment of OAB, except propiverine and oxybutynin, show only antimuscarinic effects, while propiverine and oxybutynin show mixed efficacy. Propiverine shows its spasmolytic, that is, the calcium channel antagonist effect, by blocking the L-type voltage-dependent Ca+2 ion channels in the bladder by its metabolites. Propiverine inhibits the efferent connection of the pelvic nerve by suppressing ATP-related bladder overactivity. In other words, propiverine can also inhibit the purinergic mechanism, which is another factor responsible for detrusor contraction.16
Since propiverine can be considered a weak inhibitor of cytochrome P450 (CYP3A4), significant increases in the concentrations of other drugs metabolized by this pathway are not expected with concomitant use with propiverine.12 The pharmacokinetics of propiverine were not altered in patients with severe renal impairment (CC<30 mL/min) or mild to moderate liver failure from fatty liver disease, and in elderly patients (60–85 years) compared to young healthy adults.15
In an open non-randomized parallel-group study, the pharmacokinetics and tolerability of a single dose of 30 mg propiverine were compared between healthy and male and female patients with severe renal impairment, taking into account age, body mass index, and gender. This study concluded that from a therapeutic point of view, dose adjustment is not necessary for patients with severe renal impairment.17
No dose adjustment is required with propiverine in patients with mild to moderate renal impairment. No dose adjustment is recommended in patients with severe renal impairment unless the dose exceeds 30mg/day. No dose adjustment is required in patients with mild liver failure.12 In a drug interaction study, it was emphasized that propiverine is a drug with a minor potential in terms of drug-drug interaction risk.18
A recent study in which propiverine was administered intravenously to animals did not show any significant blood pressure changes.42 In addition, Maruyama et al., in an in vivo study on rats, aimed to detect muscarinic receptor levels in tissues such as the bladder, submaxillary salivary gland, large intestine, stomach, and heart, and to evaluate the selectivity of anticholinergics to the bladder, which are used in the treatment of overactive bladder. As a result of the study, propiverine showed high selectivity to the bladder while low selectivity to heart tissue.43 In vitro and in vivo studies of propiverine to date reveal no evidence of increased cardiovascular risk.14
Yusup A et al., in an animal study, concluded that antimuscarinic agents crossed to the brain suppress the muscarinic excitatory systems in the dorsal pontine tegmentum and reduce the urinary drainage success, therefore, propiverine is a suitable option in the treatment of patients with OAB as a result of cerebral infarction since it crosses the blood-brain barrier at low rates.50
In many clinical studies, propiverine has been shown to be a statistically significant effective and safe treatment option in patients with stress incontinence, urge incontinence, and combined urge-stress incontinence.19,20
Based on the results of three studies of the effects of propiverine hydrochloride on the overactive bladder, involving 2265 participants (1227 patients in the treatment group and 1038 patients in the control group), the efficacy of propiverine in terms of urgency, urge incontinence, and nocturia was confirmed by comparison with placebo (p<0.00001). According to the results of four studies involving 2302 participants (1263 patients in the treatment group and 1039 patients in the control group), nocturia episodes experienced in the propiverine group at 24 hours were observed at a significantly lower frequency compared to the control group (p=0.03). According to the results of five studies involving 2891 participants (1648 in the treatment group and 1243 in the control group) on the effects of propiverine hydrochloride on the overactive bladder, the increase in urine volume per voiding (ml) was significantly higher in the propiverine group compared to the control group (P< 0.00001).11
In dose determination studies, it has been reported that 50% improvement was observed in the efficacy parameters in the group given 15 mg/day, while the efficacy reached 80% in the group given 30 mg/day.21 The subjective improvement in OAB patients with the use of propiverine has been shown to be 77%. In addition, a decrease in max detrusor pressure and an increase in max bladder capacity are observed compared to placebo.23 It has been reported that 12 weeks of treatment with the slow-release form of propiverine (ER 30 mg) reduces the frequency of urinary incontinence within 24 hours by 68.3%.24 It was reported that improvement in quality of life scores, frequency of voiding, and incontinence attacks was higher in the group using propiverine in a 6-week study, which was carried out in 41 female patients with the use of propiverine 15mg 2x1 and tolterodine 2mg 2x1.12
In studies with propiverine in which a total of 1622 patients were evaluated, it has been reported that the treatment groups in which propiverine was combined with α-blockers had significantly higher total international prostate symptom score (IPSS), storage symptom score, and voiding symptom score, as well as an increase in maximum flow rate, compared to the groups using only alpha-blockers.11,25
In a multicenter, randomized, double-blind study of the α1 adrenoceptor antagonist doxazosin and propiverine in patients with obstruction due to benign enlargement of the prostate and overactive bladder, voiding frequency (23.5%-14.3%, p=0.004), mean urinary volume (32.3%-19.2%, p=0.004) and patient satisfaction (p=0.002) with results from IPSS symptoms of storage (41.3% vs. 32.6%, p=0.029) and urgency (p=0.019) favored the combination group. As a result of this study, the combination of antimuscarinics and α1 adrenoceptor antagonists was shown to be effective and safe in patients with obstruction due to benign enlargement of the prostate and overactive bladder.26
The most common side effect of antimuscarinic drugs compared to placebo is dry mouth.27 Other side effects that can be observed due to antimuscarinic use are constipation, pruritus, and headache; however, no association was found between treatment with antimuscarinics and the risk of serious adverse events.27 Constipation may be the most bothersome side effect for many on antimuscarinic therapy. Constipation is particularly common in elderly patients and is a concern for the quality of life in this population, as well as an increased financial burden in terms of healthcare costs.28,29
Antimuscarinics may cause undesirable effects such as dizziness, drowsiness, and insomnia. Due to differences in lipophilicity, antimuscarinics tend to cross the blood-brain barrier and exhibit central nervous system side effects.30 As shown in previous systematic reviews, all currently used antimuscarinics have similar efficacy, and this view is supported by the NICE guidelines. Therefore, differences in adverse event profiles should guide treatment selection. If adequate effect is not observed after initiation of treatment with antimuscarinics, it is recommended to increase the dose or replace it with another antimuscarinic.9,31,32
According to the results of the review of four studies in which 2042 patients receiving propiverine hydrochloride treatment were evaluated, it was reported that there was no clinical difference in the incidence of blurred vision side effects when compared with the propiverine and control groups.11 In one study, treatment with propiverine was found to be well-tolerated and safe in patients with primary open-angle glaucoma and primary angle-closure glaucoma.33
In a study by Madersbacher et al., it was emphasized that propiverine may be a safe treatment option except for patients with angle-closure glaucoma who have not been examined for ophthalmological safety. In addition, in the same study, they examined 6 clinical studies in which patients were given propiverine for up to 12 months and followed up to 1–2.5 years, and they emphasized that the rates of adverse events were between 8.7% and 22.7% and stated that no serious side effects were encountered.13
A study was conducted in which propiverine was compared with tolterodine in terms of its effects on efficacy, tolerability, and quality of life. In this study, side effects were seen in 42 of 100 patients using propiverine, while side effects were observed in 43 patients in the tolterodine group. Dry mouth, the most common adverse effect, was observed in 20 patients in the propiverine group and in 19 patients in the tolterodine group.34
In the study published by Aloussi et al. in 2000, in which the data of 4390 patients were published, 18.1% of the patients reported dry mouth at the beginning (T0), while this rate was found to be 33.8% at the 4th week (T4), and this value decreased to 26.0% at the end of the study (T12). Among these, the rate of those who described severe dry mouth at the end of the study was reported as 0.7%. Mild dry mouth was reported as 22.2%, while moderate dry mouth was reported as 3.1%.35
In a meta-analysis study evaluating 26229 patients, of which 76% were women, with a mean age of 59 (range 30 to 82) and a mean duration of treatment of 8 weeks, they reported that all antimuscarinic drugs, including propiverine, had similar side-effect profiles, and the most common complaint was dry mouth. In addition, the study showed that age, gender, and duration of treatment did not have a significant effect on adverse events.9 While no change was detected in the side-effect profile with 12-week Propiverine treatment with the participation of approximately 7300 patients, in a 12-week clinical follow-up of 4390 patients, it was seen that the side effects observed decreased as the duration of propiverine ER 30mg use increased;12,35 whereas another study showed a linear relationship between the efficacy of propiverine and the resulting side effects.37
In the study in which 324 patients using propiverine (30 mg/day) ER and tolterodine (4 mg/day) ER for 8 weeks, the improvement in symptoms was found to be 88.9% in the propiverine group and 77.8% in the tolterodine group, and the rate of discontinuation of treatment due to side effects is also reported to be lower in the propiverine slow-release (ER 30 mg) group compared to tolterodine 4 mg ER group.38 However, in different studies comparing propiverine (30 mg/day) and tolterodine ER (4 mg/day), the frequency of adverse events was observed to be comparable in both treatment groups (Figure 1).34,40
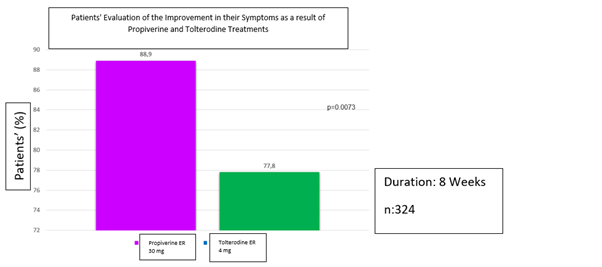
Figure 1 Patients' evaluation of the improvement in their symptoms as a result of propiverine and tolterodine treatments (Adapted from Leng J et al.38)
In two studies comparing propiverine and oxybutynin, dry mouth and severe dry mouth were observed less frequently in the propiverine administration.6,39 It has been reported that propiverine and oxybutynin exhibit similar efficacy when evaluated in terms of bladder diary variables and urodynamic parameters.
Dorschner et al. evaluated the cardiac safety of propiverine in the elderly and reported that there was no significant difference between heart rate and PQ, QRS, QT, or QTc intervals in resting and ambulatory ECGs taken throughout the study, and there was no difference between placebo and propiverine in other cardiac parameters.22
With propiverine ER 30 mg, there was no difference in QT intervals and heart rates in the patient groups aged 65 years and older.41 It has been reported that propiverine did not affect cardiac repolarization at therapeutic doses (15mgx3), where an increase in cardiac side effects is not expected at recommended therapeutic doses, and it does not raise any concerns regarding cardiac safety even in patients at risk of cardiac arrhythmia.10,12 In a study comparing elderly patients and younger patients, no clinically significant difference was found in the incidence of side effects.12
In the study, in which a total of 745 patients with OAB were included, 3 treatment groups were formed from patients using propiverine 30 and 45 mg, and drug efficacy was evaluated by monitoring the frequency of urinary urgency, incontinence, urination, and nocturia attacks experienced by the patients. Group 1 consisted of patients who started using 30 mg of propiverine and continued for 12 weeks. The second group consisted of patients who started using 30 mg of propiverine and started using 45 mg of propiverine as of the 4th week and continued with 45 mg dosing for 8 weeks. The third group consisted of patients who started using 45 mg of propiverine and continued to use 45 mg for 12 weeks. As a result of the study, patients using propiverine 45 mg have been observed to experience significantly less frequent urinary urgency, urinary frequency, incontinence, and nocturia (p<0.0001), while both propiverine 45 mg and propiverine 30 mg had similar tolerability.36
In a study comparing propiverine hydrochloride IR and propiverine hydrochloride ER formulations, both formulations of propiverine showed similar clinical efficacy, tolerability, and frequency of side effects.31 In another study by Voigt et al., it was seen that the efficacy of propiverine continued throughout the 10-year treatment of 29 female patients with urgency urinary incontinence. It has been observed that efficacy lasted for 10 years, with patients who discontinued treatment due to improvement within this period, but with equally successful results when they resumed treatment upon relapse of symptoms.44
In another study involving 1154 people who received propiverine treatment, it was observed that efficacy with propiverine was maintained for 1 year, and this efficacy increased over time.45
Propiverine has been shown to improve quality of life without affecting cognitive functions.16 In addition, Uchiyama et al. evaluated the elderly (71± 8.2 years old) and patients with neurological diseases (Multiple brain infarcts, Parkinson's disease, Alzheimer's disease) staying in nursing homes and hospitals in terms of cognitive parameters before and after propiverine treatment, and no significant changes were observed in post-void residual measures, higher brain function scales, parameters, and motor functions measured by CIBIC (Clinician's Interview-Based Impression of Change).46 Patients with detrusor hyperreflexia may be treated with an anticholinergic agent, including propiverine.47
Siegert et al. evaluated psychomotor parameters such as alertness, attention, reaction times, complex acoustic and optical reaction times in propiverine and placebo users in a prospective, double-blind, randomized, placebo-controlled, and crossover study conducted at the Psychiatry Clinic of Humboldt University in Berlin. In this study, no significant difference was found between the placebo and propiverine groups in terms of the aforementioned parameters.48
An analysis evaluating the results of 6 clinical studies with propiverine also showed that propiverine did not cause post-void residual (PVR) in patients with overactive bladder.49
The International Children's Continence Society defines overactive bladder (OAB) as "urinary urgency, usually accompanied by frequency and nocturia, with or without urinary incontinence, in the absence of urinary tract infection or other obvious pathology".51
The most common symptoms are frequent urination (85%), urgency (54%), and urinary incontinence (36%), respectively.5 This situation negatively affects self-confidence and quality of life, especially in the presence of incontinence for pediatric patients and their families. It is an important problem because it impairs the social, emotional, and behavioral development of children.52,53
According to two large-scale studies conducted in 2006 and 2009, the prevalence of OAB in children ranges from 15-20%.54,55 In studies on the prevalence of OAB in children, it has been suggested that the prevalence of OAB is between 5-12% in young ages, while this frequency decreases to 0.5% in adolescents aged 16-17 years.56,57
In 2009, in a double-blind, multicenter study conducted by 38 investigators in 6 European countries, boys and girls aged 5–10 years with a bodyweight of 17–45 kg, with a micturition frequency of ≥8/day, and ≥1 incontinence episode within 7 days, the efficacy of 20 or 30 mg/day propiverine was compared with placebo. For this purpose, propiverine was administered to 87 of 171 children and placebo to 84 of them. In the general population, propiverine showed significant efficacy compared to placebo after 8 weeks of medical treatment. An important treatment goal in children with OAB and incontinence is an observable increase in bladder volume. The bladder volume in children treated with propiverine increased approximately 6 times (+31 ml) compared to children treated with placebo (+5 ml). In addition, according to the data of the study, propiverine was well tolerated in children. Mild to moderate adverse events occurred in 95% of patients under propiverine treatment, which were similar to those treated with placebo (94%). The results of this study demonstrated superior efficacy and tolerability of propiverine in children with OAB and urinary incontinence.58
In a prospective study evaluating the efficacy and tolerability of propiverine for the treatment of neurogenic detrusor overactivity (NDO) in children, reflex volume (RV), maximum detrusor pressure (MDP), maximum cystometric bladder capacity (MCBC), and bladder compliance (BC) were monitored before and after the twice-daily propiverine hydrochloride regimen. Propiverine hydrochloride has been shown to be effective and well tolerated in the treatment of children with NDO. It is emphasized that propiverine may be a preferable option to oxybutynin in pediatric patients with NDO.59
In addition, studies using propiverine treatment in pediatric patients showed a lower incidence of dry mouth or visual impairment compared to adults.41,60
In a retrospective, observational cohort study, in which the efficacy, tolerability, and safety of propiverine in children with overactive bladder-related urge incontinence (UI) were evaluated in comparison with oxybutynin, 621 children aged 5-14 years with UI due to overactive bladder in six study centers have been included. Following anticholinergic therapy (437 propiverine, 184 oxybutynin), 61.6% of propiverine-treated patients and 58.7% of oxybutynin-treated patients achieved continence after 186 and 259 days, respectively. It was observed that propiverine exhibited significantly more favorable tolerability compared to oxybutynin at the end of the study, in which adverse drug reactions caused by propiverine or oxybutynin were recorded as 2.8% versus 9.2% respectively, and early discontinuation of the treatment due to adverse drug reactions was recorded as 1.6% versus 4.4% respectively.45
It has been reported that propiverine causes a significant increase in functional bladder capacity and a decrease in voiding frequency in children with overactive bladder disease, whose mean age is 5.9 years. In addition, while significantly improving lower urinary system symptoms in children; the overall response rate for the total treatment group was 86.8%.45
In the study, in which the data of pediatric patients over 5 years of age with a diagnosis of OAB who used propiverine between the dates of 2006 and 2011 were evaluated retrospectively, the mean daytime voiding frequency decreased from 14 to 8.5, while the mean functional bladder capacity increased from 140 ml to 150 ml with propiverine treatment. In the same study, it was reported that the response rate to treatment with propiverine was 86.8%, and a higher symptomatic improvement was observed with longer-term propiverine treatment, while only 2.9% of patients with propiverine treatment had side effects that were not interpreted as serious.61
A retrospective and observational cohort study compared the efficacy, tolerability, and safety of propiverine and oxybutynin in children and adolescents with neurogenic detrusor overactivity, in that regard, propiverine, and oxybutynin treatments were administered to 255 children and adolescents (aged 1-18 years) with neurogenic detrusor overactivity (199 myelomeningoceles, 46 spinal cord injury, 10 other diagnosed patients) in total. Patients receiving propiverine and oxybutynin treatments for at least 12 months were evaluated urodynamically and clinically before and after treatment and the percentage of patients with a decrease in Maximum Detrusor Pressure (at Pdetmax) ≤40cm H2O or half from baseline was found to be 74.2% with propiverine versus 49.6% with oxybutynin (p <0.0005).62
Propiverine has the highest level of evidence (1B/C) of all anticholinergics by the pediatric committee in the International Incontinence Consultation.41
Studies comparing the treatment of propiverine 15mg 2x1 and Tolterodine 2mg 2x1 reported that improvement in quality of life scores, frequency of voiding, and incontinence attacks was higher in the propiverine group.12 It has been reported that the treatment groups in which propiverine combined with α-blockers resulted in a statistically significant decrease in the total international prostate symptom score (IPSS), storage symptom score, and voiding symptom score, as well as an increase in maximum flow rate, compared to the groups using only alpha-blockers.11,25 Studies have shown that the combination of antimuscarinics and α1 adrenoceptor antagonists is effective and safe in patients with obstruction due to benign enlargement of the prostate and overactive bladder.26 The most common side effects associated with antimuscarinic use are dry mouth, constipation, pruritus, and headache.27-29
It has been shown that antimuscarinics may cause undesirable effects such as dizziness, drowsiness, and insomnia,30 while Propiverine has been shown to increase the quality of life without affecting cognitive functions.16 In addition, many studies have reported that propiverine may be contraindicated only in unexamined angle-closure glaucoma patients in terms of ophthalmologic safety.13 It is reported that antimuscarinic drugs have similar side-effect profiles and the most common complaint is dry mouth. However, there are studies reporting a decrease in the frequency of side effects, especially dry mouth, in long-term use of propiverine.12,35,36 In studies in which its cardiac safety was questioned, no changes were observed in cardiac parameters in patients receiving propiverine therapy.22 In studies comparing elderly patients and young patients, it was reported that there was no clinically significant difference between the two groups in terms of the incidence of side effects.12
In addition, while clinical efficacy was observed with increasing dosing in the dose determination studies of Propiverine, no statistically significant difference was observed in terms of side effects and tolerability.21 A significantly higher clinical efficacy was observed in patients using propiverine 45 mg compared to propiverine 30 mg (p<0.0001), emphasizing that both propiverine 45 mg and propiverine 30 mg had similar tolerability.36 In a study in which different formulations of propiverine, including different IR and ER, were compared, it was observed that similar clinical efficacy, tolerability, and frequency of side effects were observed,31 while it was reported that the clinical efficacy of propiverine was preserved even when used for up to 10 years.55
Statistically significant efficacy and tolerability of propiverine have been demonstrated in studies in pediatric patients with overactive bladder and urinary incontinence.58 It is emphasized that propiverine may be a preferable alternative to oxybutynin in terms of tolerability in pediatric patients with neurogenic detrusor overactivity.59
In a study, where 437 pediatric patients were treated with propiverine and 184 with oxybutynin, continence was achieved after 186 days in 61.6% of the propiverine treatment group and after 259 days in 58.7% of the oxybutynin treated group. As a result of the study, adverse drug reactions caused by propiverine or oxybutynin were recorded as 2.8% versus 9.2% respectively, and the rates of early discontinuation of treatment due to adverse drug reactions were recorded as 1.6% versus 4.4% respectively. It was observed that propiverine exhibited significantly more favorable tolerability compared to oxybutynin. While the tolerability rate, which was evaluated as “very good” and “good”, was 91.7% with propiverine, this rate was 68.3% with oxybutynin. The most common adverse reaction, dry mouth, was observed ~twice as often in those treated with oxybutynin compared to those treated with propiverine.45
In the study, which was carried out with the participation of 38 researchers from 6 European countries, propiverine treatment was applied to children with neurogenic overactive bladder and urinary incontinence, treatment with propiverine has been shown to significantly decrease the frequency of daily urination (p=0.0007) and incontinence episodes (p=0.0005); while increasing the mean one-time and weekly urinary volumes (p=0.0001).58
Pediatric and adolescent patients with neurogenic detrusor overactivity had a significantly lower Maximum Detrusor Pressure (Pdetmax) with propiverine treatment compared to oxybutynin (p=0.001). However, maximum cystometric bladder capacity (MCC) was also found to be significantly higher with propiverine treatment compared to oxybutynin (P=0.001). In the same study, overall adverse reactions were reported in ~10% of patients with propiverine and in ~26% of patients with oxybutynin, and propiverine was better tolerated compared to oxybutynin.62 Furthermore, propiverine is recommended by the pediatric committee at the International Incontinence Consultation with the highest level of evidence (1B/C) of all anticholinergics (Table 3).41
Levels of Recommendation and Levels of Evidence for Anticholinergics in the International Incontinence Society Children's Committee 2017 Guidelines |
|
Propiverine |
1B/C |
Oxybutynin |
3C |
Tolterodine |
3C |
Trospium |
3C |
Solifenacin |
2C |
Darifenacin |
- |
Fesoterodine |
- |
Table 3 Levels of Recommendation and Levels of Evidence for Anticholinergics in the International Incontinence Society Children's Committee 2017 Guidelines (Adapted from Abrams P et al.41)
According to the International Continence Consultation, it is recommended to use anticholinergics for at least 2 months, preferably 3-4 months and even longer for the symptomatic treatment of OAB in children. In comparative studies of propiverine treatment and oxybutynin, propiverine was found to be significantly more effective than oxybutynin in terms of urodynamics and patient tolerability, and a significant improvement was achieved with propiverine in Maximum Cystometric Capacity (MCC), Maximum Detrusor Pressure (Pdetmax), Bladder Compliance (BC) values.
Propiverine is significantly effective in the treatment of children with urge incontinence due to OAB. Propiverine, which has similar clinical efficacy with oxybutynin, has higher tolerability and safety compared to oxybutynin. Propiverine, which is recommended to be used for at least 2, preferably 3-4 months, for continence in children, is a treatment option that can be preferred instead of oxybutynin with its superior properties.
Propiverine is an effective and safe treatment option that can be the first choice in the symptomatic treatment of both adult and pediatric patients with overactive bladder or neurogenic detrusor overactivity, with its high efficacy and significantly higher tolerability.
None.
Kubra Saygisever-Faikoglu, Gokhan Faikoglu and Fatmanur Otmar Ozcan are medical advisors of Recordati.

©2022 Saygisever-Faikoglu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.