eISSN: 2379-6367


Research Article Volume 5 Issue 4
Department of Pharmaceutical and Biomedical Sciences, California North State University, USA
Correspondence: George Talbott, California North State University, College of Pharmacy, USA, Tel 9166867413
Received: July 11, 2017 | Published: August 29, 2017
Citation: Talbott G, Woldemariam T, Fitzpatrick LR. Effects of silymarin fractions on pro-inflammatory cytokine secretion from macrophage and colonic epithelial cell lines. Pharm Pharmacol Int J. 2017;5(4):140-145. DOI: 10.15406/ppij.2017.05.00128
Silymarin is an extract of milk thistle seeds, consisting of four major classes of flavonoligans: silybinin, silychristin, silydianin and isosilyibinin. These compounds are known for their anti-oxidant and anti-inflammatory properties. The goal of this study was to determine if different fractions of the crude extract of silymarin inhibited pro-inflammatory cytokine secretion from macrophage and colonic epithelial cell lines.
Methods: Milk thistle seeds were obtained from a commercial supplier. The powdered seeds (30g) were percolated with methanol (100mL x 3). Evaporation of the solvent at reduced pressure yielded a residue. The residue was then chromatographed on a flash chromatography system. Fractions were monitored by TLC on silica gel, as described previously, and confirmed by (LC/MS) analyses. The crude silymarin extract and six distinct fractions were tested in two different cell culture systems. The secretion of TNF-α was determined from LPS-stimulated RAW 264.7 murine macrophages. We also characterized the effects of silymarin fractions on TNF-a induced chemokine (IL-8) secretion, using a HT-29 colonic epithelial cell line.
Results: Fraction 5 (containing mainly silybinin and silychristin) was most potent for inhibiting TNF-α secretion from LPS-stimulated macrophages (IC50=47.7μg/ml). Fractions 2 and 4 (composed of mostly silybinin A and B) exhibited IC50 values of ≈65μg/ml in this assay. In contrast, the crude silymarin extract (IC50 value of 88.5μg/ml) was less potent for inhibiting TNF-α secretion. The potency order of tested fractions, for inhibiting IL-8 secretion by HT-29 cells was: Fraction 2 (IC50=19.9μg/ml) >Fraction 5(IC50=32.8μg/ml) > Crude extract (36.1μg/ml).
Summary: Silybinin and isosilybinin containing fractions of silymarin more potently inhibited pro-inflammatory cytokine secretion from macrophage and colonic epithelial cell lines.
Conclusion: These data contribute to the characterization of optimal anti-inflammatory flavonoligans, which can be used for follow-up testing in animal models of colitis
Keywords: silymarin, natural products, macrophages, ht-29 cells
LPS: Lip Polysaccharides; IBD: Inflammatory Bowel Disease; TNF-α: Tumour Necrosis Factor; RP-HPLC: Reverse-Phase High Performance Liquid Chromatography; TLC: Thin Layer Chromatography
Silymarin is an extract of milk thistle seeds. The principal components of silymarin are silibinin A, silibinin B, isosilibin A, isosilibin B, silichristin A, silichristin B and silidianin. Silibinin is generally considered to be the major and most active component in silymarin.1 The first six compounds exist as equimolar mixtures as trans-diastereoisomers. These diastereomers have very similar 1H and 13C NMR spectra and have no characteristic signals for facile identification of the individual isomers.2 These compounds are known for their anti-oxidant and anti-inflammatory properties.3 Human clinical trials have investigated milk thistle or silymarin primarily in individuals with hepatitis or cirrhosis, although small studies have been reported about individuals with acute lymphoblastic leukemia, prostate cancer, breast cancer, and hepatocellular carcinoma.4 Of note, silymarin showed positive results in a small clinical trial utilizing patients with Ulcerative Colitis.5 In an attempt to discover novel bioactive plant natural products for the potential treatment of inflammatory bowel disease (IBD), we examined Silymarin fractions that could have significant potential for containing an anti-colitis agent. Therefore, the anti-inflammatory effects of these fractions isolated from silymarin were tested for blocking cytokine production induced by lip polysaccharide (LPS) on macrophages and tumour necrosis factor (TNF-α) on colonic epithelial cells. These cell types and cytokines are thought to play an important role in animal models of colitis and IBD.6-8
Plant material
The seeds of Milk thistle, used in this investigation were obtained from a commercial supplier (San Francisco Herb Co., San Francisco, CA, USA).
Reagents and reference materials
The analytical reference standard for silibinin was purchased from Sigma. All solvents and reagents were of annular grade.
Extraction and isolation
The powdered seeds (30 g) were percolated with methanol (100mL x 3). Evaporation of the solvent at reduced pressure yielded a residue. The residue was chromatographed on a CombiFlash Companion® flash chromatography system (Teledyne Isco) (gradient, water/MEOH, 0, 100%, v/v) on RediSep Rf Gold C18Aq, 12g, at a flow rate of 30ml/min. Fractions (15 ml each) were monitored by TLC on silica gel using dichloromethane -hexane - acetone (10:5:1, v/v/v) as the mobile phase.
Experimental chemistry methods
Mass spectra were recorded on Agilent 1200 Series LC/MSD VL system. MS data were recorded on Agilent Technology (HP) instrument with 5973 Network Mass Selective Detector (MS model). All the fractions and eluates were monitored by thin layer chromatography (TLC) using precoated silica gel 60 F254, 0.25 mm aluminum backed plates (Merck). The TLC spots were detected by spraying with vanillin-H2SO4 reagent followed by heating. All chemicals and reagents used for TLC were of analytical grade. All the fractions and eluates were monitored by reverse-phase system on LaChrom Elite® HPLC System.
Thin layer chromatography analysis
Flavonolignans were analyzed by Thin Layer Chromatography (TLC). Chloroform-acetone-formic acid (75:16.5:8.5) was used as a solvent system. Silymarin is characterized under UV-365 nm by two intense green-blue fluorescent zones of silybin/isosilybin (Rf = 0.6), silychristin (Rf = 0.35).
Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC) analysis
A Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC) method was developed using water: methanol, gradient 100:0 to 0:100 % over 20 minutes for separation of the compounds. The RP-HPLC LaChrom Elite® Hitachi System is equipped with a photodiode-array detector (L-2455).
The crude silymarin extract and distinct fractions were tested in different cell culture systems, as described below.
Murine macrophage (RAW 264.7) cell line
One hour prior to stimulation of murine derived macrophages, silymarin fractions were added to the cell culture system at concentrations of 20 to 200μg/ml. RAW 264.7 murine macrophages were then stimulated with LPS (1μg/ml). After 4 hours, supernatant was collected for the determination of TNF-α, using an appropriate ELISA kit from R&D Systems Inc. (Minneapolis, MN). Cell viability was determined by the trypan blue exclusion method.
Colonic epithelial (HT-29) cell line
One hour prior to stimulation of human colonic epithelial cells, silymarin fractions were added to the cell culture system at concentrations of 20 to 100μg/ml. HT-29 colonocytes were then stimulated with TNF-α (10ng/ml). After 4 hours, supernatant was collected for the determination of IL-8, using an appropriate ELISA kit from R&D Systems. Cell viability was assessed by the trypan blue exclusion method.
Non-linear regression analyses were done with a GraphPad Prism® software program (Carlsbad, CA), in order to determine the reported IC50 values.
Chemistry data
Figure 1 demonstrates the extraction and isolation procedure for isolation of sylimarins from finely powdered Milk Thistle seeds.
Spectral data of isolated compounds
In the course of our bioassay-guided screening of compounds, a two-step fractionation afforded active compounds identified as silibinin, isosilibinin, and silychristin from the seeds of Milk thistle as confirmed by MS, TLC, HPLC, and by comparison with literature data. The purity and identity of the compounds was also examined by diode array detection and by comparison with standards using the Hitachi system. The structures of these compounds are summarized in Figure 2 & 3. The physiochemical properties of the silymarins are shown in Table 1. The spectroscopic data of silymarin fractions are shown in Table 2.
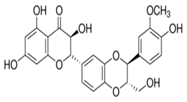
Silibinin A |
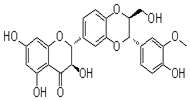
Silibinin B |

Isosilibinin A |
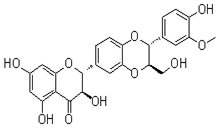
Isosilibinin B |
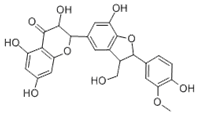
Silichristin A |

Silichristin B |
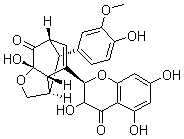
Silidianin |
||
Figure 2
Appearance |
White amorphous powder |
Molecular formula |
C25H22O10 |
HREI-MS (m/z) |
Calcd: 482.44 |
UV lmaxMeOH nm (e) |
225(s), 287 |
Table 1 Physiochemical properties of the silymarins
Silibinin: White amorphous powder. Molecular Formula C25H22O10. Molecular Weight 482.12
Isosilibinin: White amorphous powder. Molecular Formula C25H22O10. Molecular Weight 482.12Silychristin: White amorphous powder. Molecular Formula C25H22O10. Molecular Weight 482.12
Fraction |
Compound(s) |
Crude Extract |
Silibinin A & B, Silychristin, Taxifolin, Isosilibinin A & B, Apigenin |
Fraction 1 |
Silibinin A & B, Silychristin |
Fraction 2 |
Isosilibinin A & B (major, 1:3 ratio), Silibinin A & B (1:3 ratio), Silychristin (minor), Taxifolin |
Fraction 3 |
Taxifolin, Silychristin |
Fraction 4 |
Silibinin A & B, Taxifolin, Silychristin |
Fraction 5 |
Silibinin A & B (major 1:1 ratio), Silychristin (minor), Isosilibinin A & B (minor, 1:4 ratio), Apigenin |
Fraction 6 |
Silibinin A & B, Silychristin |
Table 2 Spectroscopic data of silymarin fractions
Biology data
As shown in Figure 4, fraction 5 (containing mainly silibinin) was most potent for inhibiting TNF-α secretion from LPS-stimulated macrophages (IC50 value = 47.7μg/ml). Fractions 2 and 4 (composed of mostly isosilibin, and silibinin) had IC50 values of ≈ 65μg/ml in this assay. In contrast, the crude silybinin extract (IC50 value = 88.5μg/ml) was least potent for inhibiting TNF-α secretion. The potency data for TNF-α inhibition by the various silymarin fractions is summarized in Table 3.
Fraction |
IC50 Value (μg/ml) |
Crude |
88.5 |
Fraction 1 |
109.8 |
Fraction2 |
67.6 |
Fraction 3 |
86.5 |
Fraction 4 |
63 |
Fraction 5 |
47.7 |
Fraction 6 |
195.3 |
Silibinin |
79.5 |
Isosilibinin |
> 200 |
Table 3 Raw 264.7 (Macrophage) cell line results summary
Cell viability was determined by trypan blue exclusion and no significant differences in viability were found for any of the fractions tested in Raw 264.7 cells or HT-29 cells except for Fraction 4 which significantly reduced cell viability at 100ug/ml and 200ug/ml in HT-29 cells (data not shown).Based on the data in the macrophage cell line, only certain silymarin fractions were utilized for follow-up biological testing. As shown in Figure 5, both the crude extract (left panel) and fraction 5 (right panel) effectively inhibited IL-8 secretion from the colonic epithelial cell line. For inhibiting IL-8 secretion by HT-29 cells, the potency order (IC50 values) for tested fractions was: Fraction 2 (19.9μg/ml) > Fraction 5 (32.8μg/ml) > Crude Extract (36.1μg/ml). These results are summarized in Table 4.
Fraction |
IC50 Value (μg/ml) |
Crude |
36.1 |
Fraction 2 |
19.9 |
Fraction 5 |
32.8 |
Table 4 HT-29 (Colonic Epithelial Cell) results summary
Our results show that crude silymarin extract, isosilibinin and silibinin containing fractions of silymarin extract inhibited pro-inflammatory cytokine/chemokine secretion from two different cell lines, albeit with different potencies. The cell lines were picked to represent cell types likely involved in the pathogenesis of human IBD.8-10 Of potential relevance to our study, previous investigators reported that silibinin treatment (50-200µM range) inhibited TNFα-induced NF-κB activation in HT29 cells.11 This mechanism of action could be the basis for the observed block of IL-8 secretion from this colonic epithelial cell line by silymarin derived fractions. Of note, all of the previous experimental IBD work with silymarin (or silibinin) was conducted with a trinitrobenzene sulfonic acid [TNBS] model of colitis. This is a model of human Crohn’s Disease,12,13while the initial clinical results were obtained in patients with Ulcerative Colitis.5-13 Ongoing work in our laboratory is examining the effects of silymarin derived fractions on cytokine production from colonic strips of mice with DSS colitis (a model of Ulcerative Colitis), utilizing an ex vivo system.10-14
In summary, the in vitro results from two cell lines further suggest that silymarin, or derived fractions may prove to be an effective natural product supplement, as part of the overall therapeutic approach for the treatment of IBD. Indeed pre-clinical and clinical evidence from previous studies support this possibility.3‒18 Future studies, should concentrate on which specific silymarin component(s) are most effective in other relevant pre-clinical models of IBD.10
None.
Author declares that there is no conflict of interest.

©2017 Talbott, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.