eISSN: 2379-6367


Research Article Volume 9 Issue 2
Department of Pharmacology and Chemical Biology, Baylor College of Medicine, USA
Correspondence: Pui-Kwong Chan, Department of Pharmacology and Chemical Biology, Baylor College of Medicine, Houston, Texas 77030, USA, Tel 713 798 7902, Fax 713 798 3145
Received: April 21, 2021 | Published: April 28, 2021
Citation: Chan PK, Mak E. Creation of a novel triterpenoid drug that inhibits DNA synthesis. Pharm Pharmacol Int J. 2021;9(2):64-75. DOI: 10.15406/ppij.2021.09.00329
Protoaescigenin, an aglycone of the Olean-12-en, was esterified with tigloyl chloride. Three esterification products: Tig-N, with esterification at C-24 position; Tig-R, with esterification at C-24 and C- 28 positions and Tig-S, with esterification at C-24 and C-21 were found to have cell-growth inhibition activity with human ovarian cancer cells (ES2). The IC50 values for Tig-N, Tig-R and Tig-S are 8.6±5.1µg/ml, 3.6±1.7µg/ml and 0.17±0.20µg/ml, respectively. All compounds have a common C-24 esterification, but Tig-S with the both (C-24 and C-21) esterification has the highest activity. Tig-S induces cell-death by the apoptosis and the drug-treated cells were arrested in the S-phase of cell cycles. Treatment with the esterified products of Protoaescigenin inhibits DNA synthesis in K562 cells in dose and time dependent manner. A drug-combination study of Tig-R with three anticancer drugs: Cytarabine, Camptothecin and Daunomycin, indicated that the drug effect of Tig-R is additive to the drug effect of these agents. This is the first report to show that protoaescigenin esterified with tigloyl groups inhibits DNA synthesis and inhibits cell-growth.
Keywords: tigloyl, triterpenoid, saponin, inhibit, DNA synthesis
Triterpene saponins are a diverse group of natural compounds with a pentacyclic core structure containing hydroxyl groups and carbohydrates. Triterpene saponin exhibits various functions including haemolytic, cytotoxic and anti-inflammation activities.1-6 The exact molecular drug mechanism of these saponins is not well defined.1 Our previous studies identified a group of cytotoxic triterpenoid saponin from a plant extract (Xanthoceras sorbifolia). We found that acylated groups and carbohydrates at certain positions of the triterpene core relates to its activity.7,8 To understand the Structure-Activity Relationship (SAR) of triterpene, here we employ semisynthetic methods to prepare saponins with functional groups at different positions of the triterpene core and compared their activities. We found that esterification of protoaescigenin with tigloyl groups induce cytotoxicity and inhibit DNA synthesis.
Materials
Escin was purchased from SIGMA (cat#E1378); Tigloyl Chloride was purchased from TCI America (cat#T1811). Cells (K562, ES2) were obtained from ATTC. Thymidine, [methyl-3H] (40-60Ci/mmol) was from PerkinElmer; antibodies to p53 from Santa Cruz Biotechnology; Anticancer drugs: Daunomycin (cat#8809), Ara-C (cat#1768), and Camptothecin (cat#C9911) are from Sigma-Aldrich. All Other chemicals are of reagent grade.
Preparation of acylated Protoaescigenin
Preparation of Protoaescigenin
Protoaescigenin9 was served as the starting material for esterification with tigloyl chloride. To prepare protoaescigenin, the acyl groups of Escin (its aglycone is protoaescigenin) were first removed by alkaline hydrolysis and acid hydrolysis. Alkaline hydrolysis of Escin was performed at 80oC in 1M NaOH for 4 hours (0.5 gram in 25 ml 1M NaOH), neutralized with HCl before acid hydrolysis. Acid hydrolyzed was done with 1N HCl in 50% methanol at 80oC for 5 hours. The solution was then neutralized with NaOH. The major product, E4A (protoaescigenin) was purified by FPLC before use. The average yield is 15%.
Esterification of protoaescigenin with tigloyl chloride
E4A (100mg, or 0.2mmole) was dissolved in pyridine (2ml). While stirring (at 25oC), tigloyl chloride (400ul or 3.2mMole) was added rand stirred for 1 minute. The reaction was stopped by adding 2N HCl (10 ml). Esterification products were recovered with ethyl acetate extraction. The yield of Tig-R is about 0.015% from E4A.
Purification of esterified Protoaescigenin
The esterified products were separated by HPLC (in a C18 reverse phase column which was equilibrated with 45% acetonitrile-0.05% trifluoroacetic acid (TFA)). Samples were eluted with gradient from 45% to 80% acetonitrile-0.005% TFA. The eluent was monitored at 207nm. Over 20 fractions were detected and isolated. Selected fractions: Tig-N, Tig-R and Tig-S were collected for use in this study.
Structural analysis of isolated compounds
NMR (1H, 13C. HMQC, HMBC) and HR-MS were performed as previously described.7,8 The pure compound was dissolved in DMSO-D6 with 0.05% v/v TMS (Tetramethylsilane). NMR spectra were acquired with a Bruker Advance 600 MHz NMR spectrometer in University of Houston. ESI-MS Mass spectra were recorded with the LTQ-Orbitrap system, Rice University.
Identification and verification of compounds
Structure of E4A
E4A (the de-acylated Escin, Protoaescigenin) was obtained as an amorphous white powder. It was assigned a molecular formula of C30H50O6 on the basis of its ESI/MS at m/z 529.3507 [M+Na]+ as well as its NMR spectroscopic data (Table 1). The 13C and 1H NMR spectroscopic and mass spectroscopic data of E4A is agreeable with the structure of Protoaescigenin.9,10 Therefore, it is confirmed that E4A is protoaescigenin [3β, 16α, 21β, 22α, 24, 28-hexahydroxyolean-12-ene] (Figure 1).
|
|
E4A (0) |
Tig-N (1) |
||||
|
Position |
δC, type |
δH (J in Hz) |
HMBC |
δC, type |
δH (J in Hz) |
HMBC |
|
1 |
38.03, CH2 |
0.91, d, 1.52, t |
38.21, CH2 |
0.97, t, 1.56, t |
||
|
2 |
27.11, CH2 |
1.61, m, 1.69,m |
26.76, CH2 |
1.52, br, m |
||
|
3 |
78.49, CH |
3.17, 1H, dd |
23,24 |
76.65, CH |
3.15, 1H, dd |
23 |
|
4 |
44.09, qC |
41.5, qC |
||||
|
5 |
55.33, CH |
0.75, 1H |
54.86, CH |
0.815, 1H |
||
|
6 |
18.55, CH2 |
1.35, 1.54 |
19.54, CH2 |
1.45, 1.65 |
||
|
7 |
32.66, CH2 |
1.25, 1.41 |
32.82, CH2 |
1.27, 1.44 |
||
|
8 |
40.07, qC |
39, qC |
||||
|
9 |
46.86, CH |
1.55, m |
46.1, CH |
1.56, m |
10,25,26 |
|
|
10 |
36.15, qC |
36.31, qC |
||||
|
11 |
23.08, CH2 |
1.80, m |
22.97, CH2 |
1.8 m |
||
|
12 |
121.64, CH |
5.18, 1H, t, |
9,11,14,18 |
121.64, CH |
5.19, 1H, t |
9,11,14,27 |
|
13 |
143.01, qC |
142.98, qC |
||||
|
14 |
42.00, qC |
40.75, qC |
||||
|
15 |
33.13, CH2 |
1.2, 1.62 |
27 |
33.11, CH2 |
1.21, 1.63 |
|
|
16 |
66.47, CH |
4.03, 1H, t |
66.51, CH |
4.02, 1H, s |
||
|
17 |
46.08, qC |
45.87, qC |
||||
|
18 |
39.6, CH |
2.27, dd, (14.4/4.0) |
14,17,19 |
39.27, CH |
2.29, br, m |
|
|
19 |
47.05, CH2 |
0.94, 2.35 m |
17,18,20,29,30 |
47.05, CH2 |
0.94, 2.33 m |
17,18,20,29,30 |
|
20 |
35.22, qC |
35.24, qC |
||||
|
21 |
76.66, CH |
3.79, 1H, d (9.6) |
20,22,29,30 |
76.7, CH |
3.79, 1H, d (9.6) |
20,22,29,30 |
|
22 |
73.87, CH |
3.6,1H, d (9.6) |
16,17,20,21,28 |
73.65, CH |
3.61, 1H, d (9.6) |
16,17,20,21,28 |
|
23 |
22.77, CH3 |
1.08, 3H, s |
3,4,5,24 |
22.58, CH3 |
1.06, 3H, s |
3,4,5,24 |
|
24 |
62.91, CH2 |
3.27, d, (11) |
3,4,5,23 |
66.17, CH2 |
4.16, d (12) |
4,5,23,1’’ |
|
3.83, d, (11) |
4.14, d, (12) |
|||||
|
25 |
15.6, CH3 |
0.87, 3H, s |
1,5,9,10 |
14.93, CH3 |
0.89, 3H, s |
1,5,9,10 |
|
26 |
16.2, CH3 |
0.815, 3H, s |
7,8,9,14 |
16.12, CH3 |
0.82, 3H, s |
9,14 |
|
27 |
26.65, CH3 |
1.34, 3H, s |
8,14,15 |
26.59, CH3 |
1.35, 3H, s |
8,14,15 |
|
28 |
65.05, CH2 |
2.99, 1H, d (10.4); 3.15, 1H, d (10.4); |
16,17,18,22 |
64.83, CH2 |
2.98, 1H, d (10.2); |
16,17,18,22 |
|
3.15, 1H, d (10.5) |
||||||
|
29 |
29.89, CH3 |
0.84, 3H, s |
19,20,21,30 |
29.89, CH3 |
0.838, 3H, s |
19,20,21,30 |
|
30 |
18.68, CH3 |
0.806, 3H, s |
19,20,21,29 |
18.64, CH3 |
0.806, 3H, s |
19,20,21,29 |
|
21-O-Tig |
21-O-Tig |
|||||
|
1’ |
||||||
|
2’ |
||||||
|
3’ |
||||||
|
4’ |
||||||
|
5’ |
||||||
|
24-O-Tig |
24-O-Tig |
|||||
|
1’’ |
167.25, qC |
|||||
|
2’’ |
128.24, qC |
|||||
|
3’’ |
136.6, CH |
6.77, 1H |
1’’,2’’,4’’,5’’ |
|||
|
4’’ |
11.94, CH3 |
1.78, 3H |
1’’,2’’,3’’,5’’ |
|||
|
5’’ |
- |
14.08, CH3 |
1.77, 3H |
1’’,2’’,3’’,4’’ |
||
|
28-O-Tig |
28-O-Tig |
|||||
|
1’’’ |
||||||
|
2’’’ |
||||||
|
3’’’ |
||||||
|
4’’’ |
||||||
|
5’’’ |
|
|
|
|
|
|
Table 1 NMR spectroscopic data (DMSO-d6) for compounds 0, 1, 2, and 3
|
|
Tig-R (2) |
Tig-S (3) |
||||
|
Position |
δC, type |
δH (J in Hz) |
HMBC |
δC, type |
δH (J in Hz) |
HMBC |
|
1 |
38.24, CH2 |
0.96, t, 1.56, t |
25 |
38.208, CH2 |
0.982, 1.572 |
|
|
2 |
26.77, CH2 |
1.52, br, m |
26.766, CH2 |
1.528 |
||
|
3 |
76,69, CH |
3.15, 1H, dd |
4,23,24 |
76.646, CH |
3.154 |
|
|
4 |
41.5, qC |
41.471, qC |
||||
|
5 |
54.88, CH |
0.82, 1H |
23,24,25 |
54.844, CH |
0.825 |
|
|
6 |
19.51, CH2 |
1.47, 1.65 |
19.548, CH2 |
1.471, 1.653 |
||
|
7 |
32.81, CH2 |
1.28, 1.43 |
32.781, CH2 |
1.269, 1.448 |
||
|
8 |
39, qC |
39.077, qC |
||||
|
9 |
46.1, CH |
1.55 m |
10,25,26 |
46.126, CH |
1.574 |
|
|
10 |
36.33, qC |
36.316, qC |
||||
|
11 |
22.97, CH2 |
1.79 m |
22.952, CH2 |
1.829 |
||
|
12 |
122.25, CH |
5.18, 1H, t |
9,11,14,18,27 |
122.092, CH |
5.242 |
9,11,14,18,27 |
|
13 |
142.32, qC |
142.524, qC |
||||
|
14 |
40.7, qC |
40.646, qC |
||||
|
15 |
33.56, CH2 |
1.28, 1.64 |
27 |
33.195, CH2 |
1.2, 1.61 |
|
|
16 |
66.47, CH |
4.01, 1H, s |
66.552, CH |
3.966 |
||
|
17 |
45.3, qC |
46.570, qC |
||||
|
18 |
39.9, CH |
2.41, br, m |
16,17,19 |
38.767, CH |
2.413, 2.449 |
|
|
19 |
46.59, CH2 |
0.98, 2.42 m |
46.674, CH2 |
1.029, 2.474 |
||
|
20 |
35.23, qC |
35.359, qC |
||||
|
21 |
76.5, CH |
3.84,1H, d (9.6) |
20,22,29,30 |
80.341, CH |
5.499, 5.479 |
20,22,29,30,1’ |
|
22 |
71.89, CH |
3.55, 1H, d (9.6) |
16,17,20,21,28 |
70.003, CH |
3.887 |
17,21 |
|
23 |
22.62, CH3 |
1.06, 3H, s |
3,4,5,24 |
22.588, CH3 |
1.062 |
3,4,5,24 |
|
24 |
66.17, CH2 |
4.14, 1H, d, (12) |
3,4,5,23, 1’’ |
66.185, CH2 |
4.14, d, (12) |
3,4,5,23,1’’ |
|
4.17, 1H, d, (12) |
4.17, d, (12) |
|||||
|
25 |
14.89, CH3 |
0.88, 3H, s |
1,5,9,10 |
14.941, CH3 |
0.8948 |
1,5,9,10 |
|
26 |
16.13, CH3 |
0.81, 3H, s |
7,8,9,14 |
16.124, CH3 |
0.8239 |
7,8,9,14 |
|
27 |
26.65, CH3 |
1.36, 3H, s |
8,14,15 |
26.637, CH3 |
1.3738 |
8,14,15 |
|
28 |
65.34, CH2 |
3.68, 1H, d (10.4); |
16,17,18,22,1’’’ |
63.207, CH2 |
2.996, |
|
|
3.73, 1H, d (10.4) |
3.127 |
|||||
|
29 |
29.87, CH3 |
0.86, 3H, s |
19,20,21,30 |
29.261, CH3 |
0.7195 |
19,20,21,30 |
|
30 |
18.49, CH3 |
0.85, 3H, s |
19,20,21,29 |
19.579, CH3 |
0.933 |
19,20,21,29 |
|
21-O-Tig |
21-O-Tig |
|||||
|
1’ |
167.124, qC |
|||||
|
2’ |
128.741, qC |
|||||
|
3’ |
135.588, CH |
6.732, 6.787 |
1’,2’,4’,5’ |
|||
|
4’ |
12.071, CH3 |
1.787 |
1’,2’,3’,5’ |
|||
|
5’ |
13.961, CH3 |
1.766 |
1’,2’,3’,4’ |
|||
|
24-O-Tig |
24-O-Tig |
|||||
|
1’’ |
167.24, qC |
167.260, qC |
||||
|
2’’ |
128.29, qC |
128.252, qC |
||||
|
3’’ |
136.8, CH |
6.77, 1H |
1’’,2’’,4’’,5’’ |
136.654, CH |
6.745, 6.799 |
1’’,2’’,4’’,5’’ |
|
4’’ |
11.9, CH3 |
1.78, 3H |
1’’,2’’,3’’,5’’ |
11.951, CH3 |
1.779 |
1’’,2’’,3’’,5’’ |
|
5’’ |
13.99, CH3 |
1.77, 3H |
1’’,2’’,3’’,4’’ |
14.092, CH3 |
1.768 |
1’’,2’’,3’’,4’’ |
|
28-O-Tig |
28-O-Tig |
|||||
|
1’’’ |
166.68, qC |
|||||
|
2’’’ |
128.1, qC |
1’’’,2’’’,4’’’,5’’’ |
||||
|
3’’’ |
136.5, CH |
6.77, 1H |
1’’’,2’’’,4’’’,5’’’ |
|||
|
4’’’ |
11.9, CH3 |
1.78, 3H |
1’’’,2’’’,3’’’,5’’’ |
|||
|
5’’’ |
14.08, CH3 |
1.77, 3H |
1’’’,2’’’,3’’’,4’’’ |
|
|
|
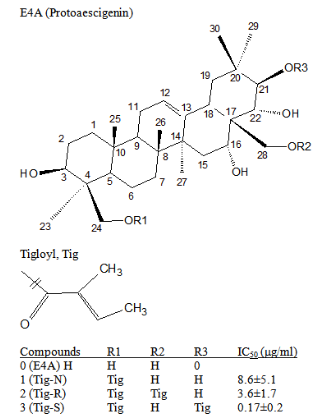
Figure 1 Chemical structure of compounds.
The chemical structure of protoaescigenin (E4A) and its tigloyl esterified products are shown. The position of tigloyl and name of these products with their IC50 values of cell-growth inhibition in ES2 cells are listed.
Structure of Tig-N
Tig-N was obtained as an amorphous white powder. It was assigned a molecular formula of C35H56O7 on the basis of its ESI/MS at m/z 589.4087 [M+H]+ as well as its NMR spectroscopic data (Table 1). The 13C and 1H NMR spectroscopic data agree that Tig-N is a protoaescigenin esterified with one tigloyl group. The assignment of C-24 is the site of esterification is based on its chemical shift shifted to downfield (δC = 66.17; δH = 4.15) as compared to protoaescigenin. This assignment was confirmed by HMBC correlation observations between H-24 (δH = 4.15) and the tigloyl carbonyl C-1’’ (δC =167.25). Based on these data, Tig-N was characterized as 24-O-tigloyl-3β, 16α, 21β, 22α, 24, 28-hexahydroxyolean-12-ene [24-O-tigloylprotoaescigenin] (Figure 1).
Structure of Tig-R
Tig-R was obtained as an amorphous white powder. It was assigned a molecular formula of C40H62O8 on the basis of its ESI/MS at m/z 671.4509 [M+H]+ as well as its NMR spectroscopic data (Table 1). The 13C and 1H NMR spectroscopic data indicated that Tig-R is a protoaescigenin with two tigloyl groups esterified at C-24 and C-28. The assignment was based on chemical shifts of C-24 and C-28, which were found shifted to downfield (for C-24, δC = 66.17; δH = 4.15; for C-28, δC = 65.34; δH = 3.7). This assignment was confirmed by HMBC correlation observations between H-24 (δH = 4.15) and the tigloyl carbonyl C-1’’ (δC =167.24); and between H-28 (δH = 3.7) and the tigloyl carbonyl C-1’’’ (δC =166.68). Based on these data, Tig-R was characterized as 24,28-O-tigloyl-3β,16α,21β,22α, 24, 28-hexahydroxyolean-12-ene. [24,28-di-O-tigloylprotoaescigenin]. (Figure 1).
Structure of Tig-S
Tig-S was obtained as an amorphous white powder. It was assigned a molecular formula of C40H62O8 on the basis of its ESI/MS at m/z 671.4501 [M+H]+ as well as its NMR spectroscopic data (Table 1). The 13C and 1H NMR spectroscopic data agree with protoaescigenin esterified with two tigloyl groups esterified at C-21 and C-24. The assignment of esterification sites was based on its chemical shifts. For C-21, it was found it shifted to downfield (δC=80.34; δH=5.49). Similar downshift was found for C-24, (δC=66.18; δH=4.15). These assignments were confirmed by HMBC correlation observations between H-21(δH=5.49) and the tigloyl carbonyl C-1’ (δC=167.124); and between H-24 (δH=4.15) and the tigloyl carbonyl C-1’’ (δC=167.26). Based on these data, Tig-S was characterized as 21,24-O-tigloyl-3β, 16α, 21β, 22α, 24, 28-hexahydroxyolean-12-ene. [21,24-di-O-tigloylprotoaescigenin]. (Figure 1).
Cytotoxic assay (MTT assay)
Cell growth activity was determined by MTT assay as described in previous publication.7,8,19 Human ovarian carcinoma cells (ES2) were used in this assay. Cells were cultured in RPMI1640 medium supplemented with 10% FCS, glutamine and antibiotics. Experiments were repeated more than three times to obtain the average IC50 (±SD) values. Daunomycin was used as a positive control and showed an IC50 of 0.13±0.01µM.
Apoptosis analysis
Human leukemia cells K562 were cultured in RPMI-1640 medium. Cells were cultured with Tig-S (0-20ug/ml) for two days. After drug-treatment, cells were collected by centrifugation, washed with PBS once, and re-suspend in binding buffer (10mM HEPES, 140mM NaCl, 2.5mM CaCl2, pH 7.4). Annexin V (Alexa Fluor 488, Invitrogen cat# A13201), and Propidium iodide (1mg/ml in water) were added to cell suspension. The mixture is incubated at room temperature for 15 minutes. Cells were washed with binding buffer 2 times before analysis by flow cytometry. Flow Cytometry was performed in Baylor Core Facility with a LSRII instrument. For reference, control cells were stained with Propidium Iodide (PI) or Annexin V-488 (Annexin V-FITC) alone. Stained cells were analyzed following Invitrogen Annexin V conjugates for Apoptosis Detection protocol. For analysis, cell distribution in following groups: live, early apoptosis, late-apoptosis, total apoptosis and dead cells were determined.
Cell-cycle phase analysis
K562 Cells (500K/ml) were cultured in RPMI-1640 medium and with drugs (Tig-S, 0-20ug/ml) for three days. After drug-treatment, cells were fixed with 70% ethanol and stained with Propidium iodide (20ug/ml)/RNase A (200ug/ml)/0.1% Triton X-100 in PBS for 30 minutes at room temperature. Flow Cytometry Analysis was performed in Baylor Core Facility with a LSRII instrument. For analysis: single cell was gated and cell-count versus Propidium Iodide (PI) signal was plotted (histogram). Cell distribution in different cell-cycle phases (G0/G1, S, G2/M) was determined.
DNA synthesis determination
Inhibition of DNA synthesis was determined follows the method described by Siegers et al.,20 in which 3H-Me-Thy (thymidine) was used as tracer. K562 cells, Human chronic MyelogenousLeukemia (CML), are grown in RPMI-1640. Cells (400K/ml) in log-growth phase were exposed to either drug or DMSO (drug solvent served as control) and 3H-Me-Thy (1uCi/ml) for 0, 0.5, 1, 2, 3 and 4 hours (Time studies). For dosage studies, cells were incubated with different concentrations of drugs (1.25–20ug/ml) for 2 hours. At the end of incubation, cells were precipitated by Trichloroacetic acid (TCA), in ice for 15 minutes. The precipitate was collected by centrifugation, and the pellet was washed two times with TCA. The pellet was dissolved in NaOH (1N) and then neutralized with HCl (2N) before dissolved in scintillation fluid. The radioactivity of sample was determined by liquid scintillation counting.
Determination of combined effects of Tig-R and anticancer drugs on inhibition of DNA-synthesis
To select a proper drug range (between 40-60% inhibition) for the combination study, a dosage curve of individual drug was first constructed before use. K562 cells were treated with drugs and radioisotope tracer (3H-Me-Thy (1uCi/ml)) simultaneously and were incubated for 2 hours. The 3H-Me-Thy incorporated into DNA was determined as described in previous section. The % inhibition is calculated as 1-% synthesis.
Determination of hemolytic activity
The hemolysis test is same as described by Voutquenne et al.,23 except that human erythrocytes instead of sheep erythrocytes were used. Erythrocytes (RBC) were isolated from human blood (EDTA whole blood, collected randomly from Texas Gulf Coast Regional Blood Center) through Ficoll-Paque gradient centrifugation. RBC were washed three times with PBS before used. 50µl of the 10% RBC suspension was added to 2ml of PBS with various concentrations of drug. The mixture was vortexed briefly and sat for 60min at room temperature. The mixture was spun at 3K for 10min (table-top centrifuge) and the lysed hemoglobin in the supernatant was measured at 540nm.
Preparation of compounds and structure analysis
Protoaescigenin is the aglycone of one of the olean-12-en in which it has six OH- groups at 3β, 16α, 21β, 22α, 24β and 28 positions.9 It was used as the starting material (Figure 1) for preparing the testing compounds. Protoaescigenin (E4A), was prepared from Escin and was then esterified with tigloyl chloride (see Experimental Section). The esterified products were separated with HPLC. About 20 fractions were obtained. Active fractions were collected and further purified. The chemical structure of these compounds was determined by NMR (1H, 13C. HMQC, HMBC) and HR-MS analysis (see Experimental Section and Table 1). Three products, compounds 1-3 (Tig-N, Tig-R and Tig-S) were selected in this study (structures are shown in Figure 1). Compound 1 (Tig-N) is a protoaescigenin with one tigloyl group at C-24 position. Compound 2 (Tig-R) is a protoaescigenin with two tigloyl groups at C-24 and C-28 positions. Compound 3 (Tig-S) is a protoaescigenin with two tigloyl groups at C-24 and C-21 positions.
Cell-growth inhibition activity of esterified compounds
The cell-growth inhibition activity by these compounds was determined in human ovarian carcinoma cells (ES2) with MTT assay. Figure 2 shows the cell-growth activity at different concentrations of esterified compounds. E4A, the starting compound without esterified group shows no activity. Activity was found in esterified products. Their IC50 values were determined. Tig-N, with one tigloyl esterification at C-24 position shows inhibition activity with the IC50 value of 8.6±5.1µg/ml. Tig-R has two tigloyl groups at C-24 and C-28 positions shows a higher activity, with the IC50 value of 3.6±1.7µg/ml. However, Tig-S, which has two tigloyl groups at C-24 and C-21 positions exhibits a much higher potency. The IC50 value of Tig-S is 0.17±0.20µM. All compounds have a common C-24 acylation, but Tig-S with an additional C-21 acylation has the highest activity.

Figure 2 Inhibition of cell growth by compounds E4A, Tig-N, Tig-R and Tig-S.
Compounds were tested for its effects on cell-growth inhibition activity in human ovarian carcinoma cells (ES2) with MTT assay. E4A: Protoaescigenin, the starting compound without esterified tigloyl group, has no inhibition activity (-⧳-). Tig-N: with one tigloyl esterified at C-24 position in protoaescigenin shows weak inhibition activity (-⧯-). Tig-R: which has two tigloyl groups at C-24 and C-28 positions shows higher activity with the IC50 value about 3.5µg/ml (-▲-). Tig-S: which has two tigloyl groups at C-24 and C-21 positions is most potent (-⧱-) with IC50 value less than one.
Inhibition of cell growth by Tig-S involves Apoptosis
To understand more of the cell-growth inhibition effect, apoptotic cells after the Tig-S treatment was determined. K562 cells were treated with Tig-S for two days and the percentage (%) of apoptotic cells was determined by flow cytometry (see methods). The results are shown in Figure 3.
As shown in Figure 3 Experiment A, the background level (no drug) of the total apoptotic cells is about 10%. With Tig-S (2.5ug/ml), the total apoptotic cells increase (from 10.5 to 15.5%). There is no increase of dead cells (level remains 1.5%). In Experiment B, which used higher concentrations of Tig-S (5, 10 and 20ug/ml), the total apoptotic cells population increased in a dose dependent manner (27% at 20ug/ml). It was noted that at higher drug concentrations, the dead cell population was also found increased (to 6%). In general, these results showed that the apoptotic cells population increase with drug-treatment and suggested that Tig-S induces cell-death by the apoptosis mechanism and not by necrosis mechanism.
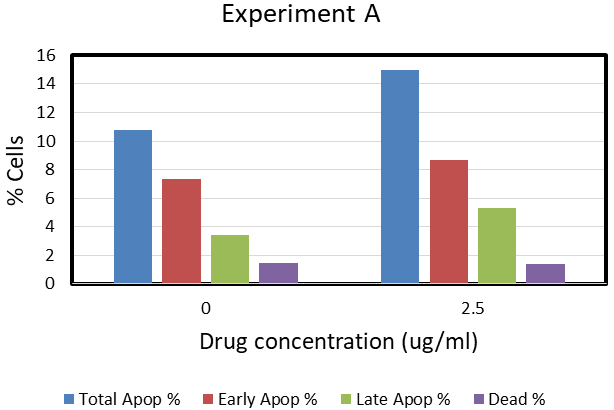

Figure 3 Apoptosis in K562 cells after Tig-S treatment.
Apoptosis was observed in K562 cells after the Tig-S treatment. K562 cells were treated with Tig-S for two days. As shown in Experiment A, after a treatment of Tig-S (2.5ug/ml), the total apoptotic cells increase from 10.5 to 15.5%. In experiment B, which used higher concentrations of Tig-S (5, 10 and 20ug/ml), the total apoptotic cells population increased in a dose dependent manner (27% at 20ug/ml). These results showed that the apoptotic cells population increase with drug-treatment and suggested that Tig-S induces cell-death by the apoptosis mechanism and not by necrosis mechanism.
Cells are arrested by Tig-S in the S-phase of cell-cycle
To learn more about the drug effect, cell-cycle phases (G0/G1, S, G2/M) analysis after Tig-S-treatment was conducted. K562 Cells were cultured with Tig-S (0-20ug/ml) for three days. The distribution of cells in different cell-cycles phases at the end of treatments was analyzed by Flow Cytometry (see method section). As shown in the PI-histogram plot (Figure 4), control cells, and cells treated with up to 0.3ug/ml of Tig-S show similar distributions of cell-cycle phases (G0/G1, S and G2/M). However, the distribution of the G2/M phase cells decrease with the increase of Tig-S. The increased effect (or decrease of G2/M peak) is observed from 0.6ug/ml to 20ug/ml while the cells distributions in G0/G1 and S phases remained relatively unchanged. These results suggested that cells were arrested in the S-phase and unable to populate into the G2/M phase of the cell cycle.

Figure 4 Cells are arrested by Tig-S in the S-phase of cell-cycle.
Cell cycle phases analysis was determined after Tig-S treatment. The PI (Propidium Iodide) histogram plot (x-axis: Propidium Iodide signal intensity; y-axis: Cell count). K562 cells were treated with Tig-S for three days. Cells were treated with Tig-S from 0 to 20ug/ml. The Tig-S concentration (in ug/ml) is listed inside each PI histogram plot. The locations of G0/G1, S and G2/M phases of cells are depicted in the PI plot of 0.15ug/ml. By comparing with these plots, it shows that the distribution of the G2/M phase cells decrease with the increase of Tig-S. The effect is observed from 0.6ug/ml to 20ug/ml. These results suggested that cells were arrested in the S-phase and unable to populate into the G2/M phase of the cell cycle.
Inhibition of DNA synthesis by Tig-S and Tig-R
To investigate that whether cell-growth inhibition by these compounds is due to inhibition of DNA synthesis, DNA synthesis in cells with drug-treatment was determined. K562 cells were treated with Tig-S for various times and dosages. The DNA synthesis in cells was determined with the radioactive tracer, 3H-Me-Thy (thymidine).20 As shown in Figure 5A (a time study), the DNA synthesis without drug (DMSO control) show an increase of 3H-Me-Thy incorporation with time. However, in the presence of Tig-S (20ug/ml), the DNA synthesis was inhibited. At the end of three hours of incubation, the DNA synthesis is 7% of the DMSO-treated control (representing 93% inhibition). In the dosage studies (Figure 5B), K562 cells were incubated with Tig-S (2.5–20ug/ml) for two hours. It was found that Tig-S inhibits DNA synthesis is dosage dependent. With the highest dosage of 20ug/ml, less than 5% of DNA synthesis was observed. Based on these results, it is concluded that Tig-S inhibits DNA synthesis in K562 cells in a time- and dosage-dependent manners. Similar results were obtained with the Tig-R treatment (Figure 5C and 5D).
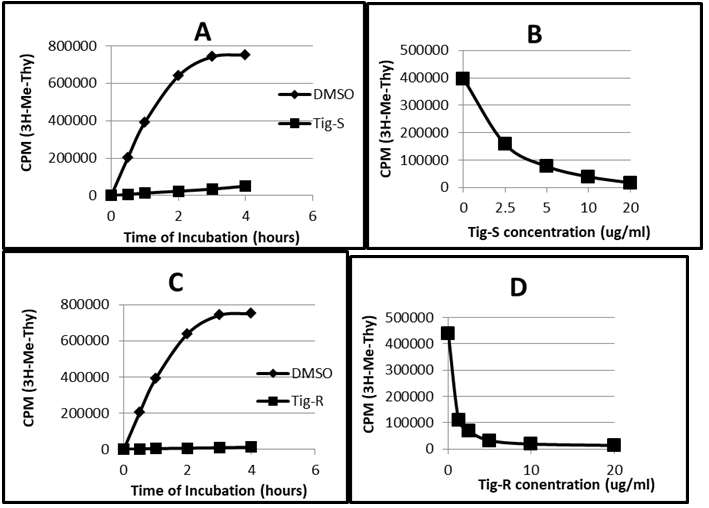
Figure 5 Inhibition of DNA synthesis by Tig-S and Tig-R in K562 cells.
5A. A time study of DNA synthesis in K562 cells. The DNA synthesis without drug (DMSO control) show a linear increase of 3H-Me-Thy incorporation (proportional to DNA synthesis) with time. In the presence of Tig-S (20ug/ml), the DNA synthesis was inhibited. At the end of three hours of incubation, the DNA synthesis is 7% of DMSO control (representing 93% inhibition). Figure 5B shows a dosage study. K562 cells were incubated with Tig-S (2.5–20ug/ml) for two hours. It shows that Tig-S inhibits DNA synthesis is dosage dependent. With the highest dosage of 20ug/ml, less than 5 % of DNA synthesis was observed. Similar results were obtained for Tig-R. Its results are represented in Figure 5C and 5D.
Combination drug effect of Tig-R with anticancer drugs
There are many anti-cancer drugs that inhibit DNA-synthesis. Whether Tig-R/Tig-S has a similar mechanism, or it can interfere or compete with these drugs is not known. In the following drug combination experiments, Tig-R is mixed with three anticancer drugs that have distinct inhibition mechanisms in DNA synthesis. These anticancer drugs are: (A) Cytarabine (Ara-C), an antimetabolite, its main drug mechanism is inhibition of DNA polymerase activity and interferes DNA elongation.21 (B) Camptothecin, a plant alkaloid, its main drug mechanism is inhibition of Topoisomerase I activity during DNA synthesis.21 (C) Daunomycin, an antibiotic, its drug mechanism includes forming complex with Topoisomerase II and causes DNA breaks.21 The results of these studies are summarized in the followings.
Combined effect of Tig-R and Ara-C
Figure 6A shows that 0.125uM of Ara-C (A1) or 1uM of Tig-R (R1), inhibited 58% or 57%, respectively, of DNA synthesis. When combined A1 and R1 (A1+R1), the DNA synthesis inhibition is 73% which is more than the inhibition by single drug A1 (58%) or R1 (57%). Results indicate an additive effect between these two drugs. Similar results obtained with double amounts of A2 and R2. The combined inhibition (A2+R2) is 87%, which is more than the inhibition by double amounts of single drug (73% for A2 or 76% for R2).
Combined effect of Tig-R and Camptothecin
In this experiment, the Tig-R (R) was combined with two concentrations of Camptothecin (C1 and C2). Figure 6B shows that Tig-R (1uM) inhibits 56% of DNA synthesis. Camptothecin 0.15uM (C1) or 0.3uM (C2) inhibits 63% or 75%, respectively, of DNA synthesis. When combining Tig-R (1uM) with either 0.15uM or 0.3uM of Camptothecin, the DNA synthesis is inhibited 77% or 84%, respectively. Both combined effects are higher than corresponding single dose of individual drug (R1, C1 and C2). The combined effect of Tig-R and Camptothecin (R1+C1) is similar to the double dose of Camptothecin (C2). Results indicate that the effect of Tig-R and Camptothecin is directly additive.
Combined effect of Tig-R and Daunomycin
Figure 6C shows the combined effects of Tig-R and Daunomycin on DNA synthesis in K562 cells. Two concentrations of Daunomycin: 0.62uM (DA1) or 1.25uM (DA2) inhibits 55% and 77%, respectively, of DNA synthesis. Two concentrations of Tig-R: 0.6uM (R1) or 1.2 uM (R2) inhibits 53% and 77%, respectively, of the DNA synthesis. The combined DA1 and R1 (Mix1) inhibits 63% DNA synthesis which is higher than single drug alone (53-55%). Results indicate additive effects. But the combined inhibition is less than the effect by the double dose of single drug (R2 or DA2) (77%). The reduced combined effect (Mix1) as compared to that of double dose of individual drug (R2 or DA2) indicate that these two drugs may compete with a common intermediate target.
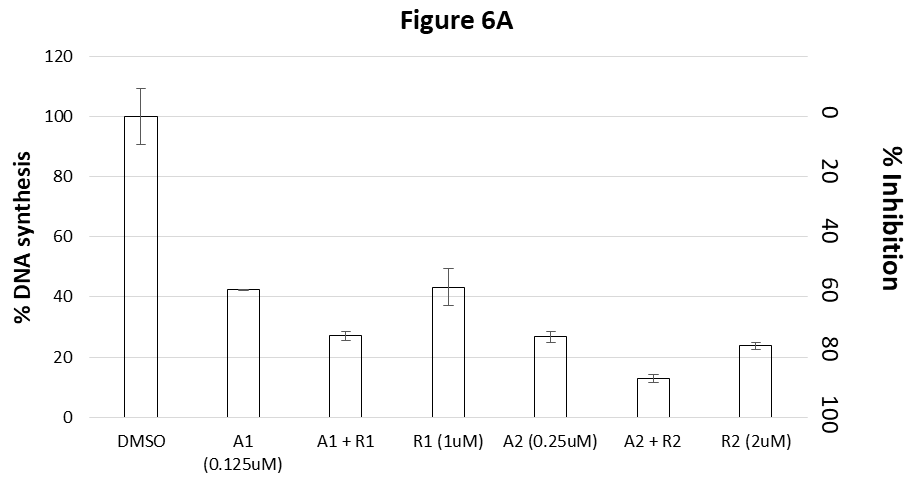
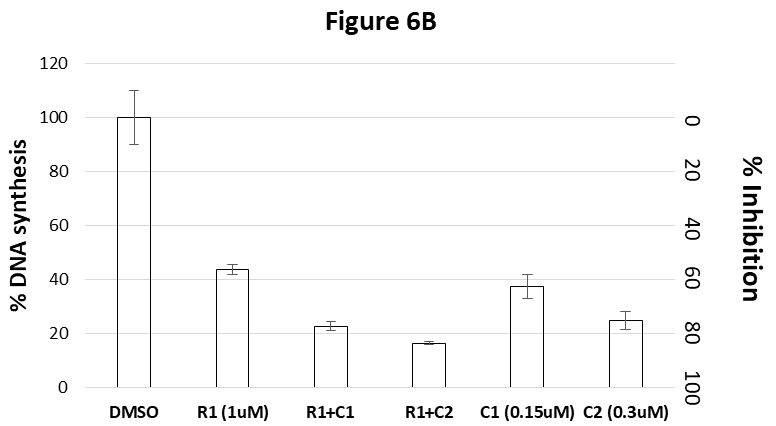
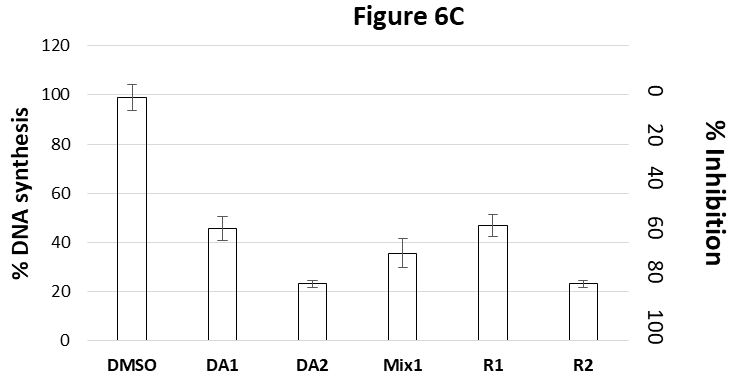
Figure 6 Combined drug effect of Tig-R with anticancer drugs.
The results of DNA synthesis in K562 cells after drug-treatments are presented. The y-axis on left: % DNA synthesis. The y-axis on right: % DNA synthesis Inhibition (% inhibition = 1 – % synthesis).
Figure 6A showed the combined effects of Ara-C and Tig-R on inhibition of DNA synthesis in K562 cells. 0.125uM of Ara-C (A1) or 1uM Tig-R (R1), inhibits 58% or 57%, respectively, of DNA synthesis. When combining (A1 + R1), the DNA synthesis inhibition is 73%, which is more than A1 or R1 alone. The combined effect is equal or similar to that of double dose of individual drug (A2 inhibits 73% or R2 inhibits 76%). Additive effect was also observed with double dose of A and R. (compare A2 and R2 with A2+R2 which inhibits 87%).
Figure 6B shows the combined effects of Camptothecin and Tig-R. Tig-R (R1=1uM) inhibits 56% of DNA synthesis. Camptothecin C1 (=0.15uM) or C2 (=0.3uM) inhibits 63% and 75%, respectively. Combining 1uM Tig-R (R1) with either 0.15uM Camptothecin (C1) or 0.3uM Camptothecin (C2), their inhibitions are 77% or 84%, respectively. Both combined effects are higher than the corresponding single dose of individual drug (R1 or C1 or C2). These results indicate that the combined effect of Tig-R and Camptothecin is additive.
Figure 6C shows the combined effects of Tig-R (R) and Daunomycin (DA) on DNA synthesis in K562 cells. Two concentrations of Daunomycin: 0.62uM (DA1) or 1.25uM (DA2) inhibits 55% and 77%, respectively, of DNA synthesis. Two concentrations of Tig-R: 0.6uM (R1) or 1.2 uM (R2) inhibits 53% and 77%, respectively, of the DNA synthesis. The combined DA1 and R1 (Mix1) inhibits 63% DNA synthesis which is higher than single drug alone (53-55%). Results indicate additive effects. But the combined inhibition is less than the effect of the double dose of single drug (R2 or DA2) (77%). The reduced combined effect as compare to that of double single dose of individual drug may indicate that two drugs compete with a common intermediate target.
Hemolytic activity of tigloyl protoaescigenin
Hemolytic activity is usually found in saponin. To determine whether the tigloyl protoaescigenin has hemolytic activity, Tig-N, Tig-R and Tig-S were mix with human red blood cells and the degree of hemolysis was determined. Figure 7 show the percentage of hemolysis versus the drug concentrations in the reaction mixture. Escin was used as a positive control (which has carbohydrates at C3 and tigloyl/angeloyl at C21, and acetyl at C22) and it has hemolytic activity starting with 0.25ug/ml. 100% hemolytic activity was observed at 5ug/ml. The results show that protoaescigenin(E4A), Tig-N and Tig-R, do not have hemolytic activity up to 20ug/ml. However, Tig-S has about 10% hemolytic activity at 20ug/ml.

Figure 7 Hemolytic activity of tigloyl protoaescigenin.
The percentage of hemolysis versus the drug concentrations were precented. Escin, a saponin, was used as a positive control. The results show that protoaescigenin ( E4A), Tig-N and Tig-R, have minimum hemolytic activity up to 20ug/ml. However, Tig-S has about 10% hemolytic activity at 20ug/ml.
Our studies indicate that the cell-growth inhibition by Tig-R/Tig-S is resulted from inhibition of DNA synthesis. But by what mechanism is not known. DNA synthesis may be affected by many reasons such as inhibition of enzymes (DNA polymerase, DNA-topoisomerases), DNA breaks or drug-binding to DNA. The possibility of DNA breaks is not likely because we found that the level of p53 is not changed in Tig-S-treated cells (data not shown). To investigate if Tig-R/Tig-S affects certain enzymes for DNA-synthesis, we employed a drug combination study. The results with Ara-C and Camptothecin show that the combined effect is a direct additive of two drugs. Because the combined effect is about equal to those of using a double amount of individual drug. These results also indicate that the Tig-R’s action is independent to those by Ara-C or Camptothecin. However, a slightly different results were obtained from mixing Tig-R and Daunomycin. While the combined effect is more than the single dose of individual drug, it is less than those of double doses of individual drug (see DA2 and R2 in Figure 6C). The reduced effect of combining two drugs as compare to the double dose of single drug indicate that the drug action of these two drugs may compete to each other or share a common intermediate target. Daunomycin has a rigid planar tetracycline ring that can intercalate DNA. Similarly, triterpene has a pentacyclic ring structure which is also rigid and planar. It is possible that these two drugs compete with each other for intercalation of DNA.
Saponin is a natural heterogeneous group of compounds that have a wide range of biological and pharmacological activities,1-6 including anti-cancer and anti-inflammatory activities.13-18 However, the precise mechanism of these saponins is not well defined. Our previous studies with the purified natural saponin show that the cytotoxic activity of these compounds depends on functional groups and its positions in the triterpene core. Because of the heterogenous nature in these compounds, it is difficult of pinpointing the significance of the active functional groups in these natural products. Our strategy to delineate the Structure-Activity-Relationship (SAR) of these compounds is to prepare compounds with identical functional groups at defined positions and compare its activity. The current studies found that the addition of tigloyl groups on the side chains, either on C24 alone, or with C24+C28, or with C24+C21 induce inhibition of cell-growth. We found that higher activity is on the combination of C24 and C21 positions. This result corroborates with the previous studies that found higher activities in compounds with acylation at C21-22 positions.7 The reasons for higher activity with C21-acylation is not known and is currently under investigation.
Haemolytic activity is a major impediment for the saponin drug development. Haemolytic activity found in saponin is associated with its carbohydrates22. We tested the hemolytic activity of the acylated compounds and found that without the carbohydrates, the hemolytic activity is largely eliminated (Figure 7). As such, this may be a useful approach to eliminate the unwanted hemolytic activity found in many saponin drugs.
In summary, we show that with this semi-synthetic method, a protoaescigenin esterified with tigloyl was produced which inhibits cell-growth. This is a first report to show that triterpenoid saponin inhibit DNA synthesis. The tigloyl esterified protoaescigenin (Tig-R, Tig-S) are useful for anticancer therapy.
The authors thank Dr. Yongcheng Song (Department of Pharmacology and Chemical Biology, Baylor College of Medicine) for suggestions and reviewing this manuscript. The authors also thank Pacific Arrow Inc., Ltd., Hong Kong, People’s Republic of China, for financial support for this project.
Authors declare that there is no conflict of interest.

©2021 Chan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.