eISSN: 2379-6367


Creative Review Volume 9 Issue 1
1Pharmacology Department, School of Pharmacy, Gannan Medical University, China
2Faculty of Medicine and Biomedical Sciences, The University of Yaounde I, Cameroon
Correspondence: Alexis Zoa, Pharmacology Department of Gannan Medical University, Jiangxi 341000, China, Tel 13182715002
Received: November 29, 2020 | Published: January 20, 2021
Citation: Zoa A. Composition and basic techniques in pharmacology laboratory: example of in-vitro study of cytotoxic drug candidates. Pharm Pharmacol Int J. 2021;9(1):1-5. DOI: 10.15406/ppij.2021.09.00317
The minimum biosafety level of pharmacology laboratory for in-vitro study of cytotoxic drug candidates must be level 2. To ensure good quality results, the pharmacology laboratory must be equipped of specified material, and researchers must have good laboratory techniques. Globally, we can divide the material for in-vitro studies of drug candidates on cancer cell lines into two parts: the material outside of the culture room and the material inside the culture room. We resume the basic techniques for in-vitro studies of candidates and cytotoxic drugs on cancer cell lines into different steps. First is the preparation step (prepare phosphate-buffered saline; prepare the incubator solution; clean the culture room; prepare the complete culture medium); second is thawing cells; third, check that the cells attached to the bottom of the petri dish or culture flask; fourth, subculture; fifth, cell count; sixth, cryopreservation; seventh, Proliferation and toxicity assays.
Keywords: biosafety, pharmacology lab, in-vitro, cells
OD, optical density (nm); FBS, fetal bovine serum; PBS, phosphate-buffered saline; SD, standard deviation; CCK-8, cell counting kit 8; DMSO, dimethyl sulfoxide; ATCC, American type culture collection; EDTA, ethylenediaminetetraacetic
Pharmacology can be defined as the study of the actions, uses, mechanisms, and adverse effects of drugs.1 A drug is any natural or synthetic substance that alters the physiological state of a living organism. So, pharmacology is an experimental science. Oswald Schmiedeberg, 1838–1921, is considered as the father of modern pharmacology.2 In 1869, Schmiedeberg showed that muscarine evoked the same effect on the heart as electrical stimulation of the vagus nerve. In 1878, he published a classic text, Outline of Pharmacology. Schmiedeberg trained most of the men who became professors at other German universities and in several foreign countries.3 Today, there is a pharmacology department in every college of medicine or pharmacy. Experimental pharmacology is done in-vitro and in-vivo. In vivo studies are done in animals, and intact animals are essential for the acute, subacute, and chronic toxicity tests that a new drug substance must undergo, and for important special tests such as teratology and carcinogenicity. Extensive studies of organ toxicity often led researchers to sacrifice animals. Animal welfare organizations oppose animal sacrifices, and in-vitro studies are increasingly prioritized in pharmacological research. The lack of public information on the composition of a pharmacology laboratory for in-vitro studies in the world in general and particularly in Africa has led us to put together this article which is intended to be original and whose main objective is to provide to the heads of pharmacology departments of African universities in general and sub-Saharan Africa in particular, public information on the equipment of a pharmacology laboratory in order to better equip them, allowing us to carry out pharmacological research on medicinal plants because we believe that Africa has great biodiversity which deserves to be exploited. The information included in this article comes from our personal experience in a pharmacology laboratory in China. We will therefore talk about the equipment of a pharmacology laboratory as well as the basic techniques common to in-vitro studies of cytotoxic drug candidates.
There are four categories of biosafety ranging from low to high risk (Table 1). Bio-hazard materials include human pathogens (bacteria, fungi, viruses, parasites, prions); all human blood products, tissues, and body fluids; cultured cells, toxins, and infected tissues.4
|
Safety level |
Risk |
Description |
Safety requirements |
|
Biosafety |
low |
Laboratories appropriate for training and teaching. |
Sink for hand washing |
|
Level 1 |
Performed with defined and characterized strains of viable micro-organisms not known to cause any disease in healthy adult humans. |
||
|
Biosafety |
Moderate |
Laboratories appropriate for diagnostics and teaching. |
Primary barriers: Face protection, gowns, |
|
Level 2 |
Performed with agents that are associated with human diseases (micro-organisms like Hepatitis B, HIV, most bacteria) as well as human body fluids, tissues, and primary human cell lines. |
Secondary barriers: Sinks for hand washing, waste decontamination facilities |
|
|
Biosafety |
Moderate– |
Laboratories appropriate for diagnostics, teaching, research, or production facilities. |
Primary barriers: Aerosol-tight chamber for work. |
|
Level 3 |
High |
Performed with exotic agents with a potential of respiratory transmission which may cause serious and potentially lethal infections (Mycobacterium tuberculosis) |
Secondary barriers: |
|
Controlled access |
|||
|
to the laboratory |
|||
|
Biosafety |
High |
Laboratory appropriate for research |
Primary barriers: complete full-body air-supplied, positive pressure personal suit (Biosafety Class III cabinets) |
|
Level 4 |
Performed with dangerous and exotic |
Secondary barriers: |
|
|
agents that pose a high individual risk |
Complete isolated |
||
|
of life-threatening disease, which is |
zone in a separate |
||
|
transmitted via the aerosol route and |
building |
||
|
for which there is no vaccine or therapy |
|||
|
|
|
available (Marburg virus, Ebola virus) |
|
Table 1 Categories of biosafety and risk levels
Recommended work practices, such as the use of appropriate labware, aseptic culture techniques, maintaining a clean working environment, as well as general hygiene (e.g., hand washing, tying back long hair, not eating, drinking, or smoking in the laboratory), are necessary to ensure safety in the lab.
This section deals with the material commonly used in a pharmacology laboratory for in vitro tests, the most common brands (without advertising), and their role. A culture room can be defined as the room keeping or incubating the culture undercontrolled temperature, light, and humidity. The culture room is also fitted with double doors in order to make it dust free and to maintain a constant room temperature.5 One should enter the culture room keeping the shoes outside the door. To maintain the temperature around 25±2°C insides the culture room, air coolers are used. The following table (Table 2) present material in two sections, material outside of the culture room and material inside the culture room.
|
Name |
Brand |
Purpose |
|
Outside of the culture room |
||
|
Autoclave |
PHCbi® |
Kill harmful bacteria, viruses, fungi, and spores on items that are placed inside a pressure vessel |
|
Etuve |
Thermo Scientific® |
heating and drying of equipment after autoclave sterilization |
|
Absorbance machine |
Varioskan Lux® |
To measure absorbance |
|
Nitrogen Transfer Vessel |
Thermo Scientific® |
Allows storage at very low temperature (-130) and over the long term (several years) of cell lines. |
|
Water bath (with adjustable temperature) |
JoanLab® |
keep materials warm over a period of time |
|
Water purification system |
Direct-Q® 5 UV |
Provide pure and ultrapure water |
|
Precision scale |
Mettler toledo® |
weigh reagents whose weight varies between 10-4 and 220 g |
|
Fridge, freezers (4°C, -20°C, -80°C) |
Midea® |
store reagents and material |
|
Glassware (2L, 1L, 500 mL, 250 mL, 100 mL, 50 mL, 25 mL) |
Pyrex® |
Transport liquids |
|
Centrifuge tubes (50 mL, 15 mL) |
Corning® CentristarTM |
Centrifugation and short-term storage of liquid that we prepared for an experiment. |
|
Pipettes and pipettors |
Axygen® and DragonLab® |
To aliquot different volumes |
|
White coat, surgical masks, care gloves and skullcap |
Protect researcher |
|
|
Chemical reagent |
Preparation of solutions |
|
|
Inside of the culture room |
||
|
Biosafety cabinet |
Thermo Scientific® |
Create sterile work surface; |
|
Humid CO2 incubator |
Thermo Scientific® |
Provide a physiological environment for cellular growth |
|
Inverted light microscope |
Leica® |
Assess cell morphology and count cells |
|
Computer (screen + central unit) |
Dell® |
project what we see under the microscope on a screen |
|
Fridge, freezers (4°C, −20°C), |
Midea® |
Store cell material, and culture components |
|
Centrifuge |
TDZ5-WS |
Condense cells |
|
Pipettes and pipettors |
Axygen® and DragonLab® |
Aliquot different volumes |
|
Cryopreservative vials tubes (1,8 mL) |
Cryopreservation of cells |
|
|
Cell media and supplementary components |
ATCC® |
Culture cells in desirable components |
|
Hemacytometer |
Count cells, determine growth kinetics and prepare suitable plating densities |
|
|
BioTech Automated Cell Counter |
Countstar® |
Count cells, determine growth kinetics and prepare suitable plating densities |
|
Cell culture dishes |
Corning® |
Culture cells in different formats (flasks, Petri dishes, 96-well plates) |
|
Containers for waste (biohazardous) |
To correctly dispose of waste |
|
|
UV light |
Maintain aseptic environment |
|
|
Alcool 70° |
To sprinkle on everything that goes in culture room |
|
|
Alcool 95° |
keep the cabinet flame alive |
|
|
Biofilm |
|
cover the tubes before storing them in the refrigerator |
Table 2 Name, brand and purpose of material for pharmacology in vitro studies
Recommended laboratory equipment for quality results of in-vitro studies of plants extracts on cancer cell lines.
Suppose we want to test a plant extract known for its anticancer activities on HepG2 hepatic carcinoma cells. The first thing to do is to culture these cancer cells so that we have a large quantity to carry out our tests. We will describe a protocol in seven steps.
Note: Wear a white coat, mask, gloves, and cap before any handling in the laboratory.
First step: Preparation
Note: Autoclave all material to be used in the culture room. Namely: micropipettes, flasks (filled with 2/3 purified water), stainless steel basin of the incubator...
Second step: thawing cells.
Note: Common procedure after any manipulation in the culture room. Close all the tubes and vials used and cover them with parafilm; Spray alcohol 70 and place the tubes and bottles in the fridge (4°C); Clean the biosafety cabinet with alcohol 70 and cotton; Extinguish the flame and close the biosafety cabinet; Turn on the biosafety cabinet UV; Take out the trash and anything that can no longer be used through the window intended for this purpose; Leave the cell room and turn off the lights; Record the handling done in a register and store the clothes and shoes used in the intended location; Turn on the UV in the cell room and the small transaction room and leave.
Third step: check that the cells have attached to the bottom of the petri dish or culture flask.
Fourth step: subculture. If confluence ≥ 80%, under biosafety cabinet:
Follow the common procedure after any manipulation in the cell room.
Fifth step: cell count.
Sixth step: cryopreservation. The cells to be cryopreserved must be counted beforehand. It is important to make sure that the cells are in the exponential growth phase before cryopreserving them.
Seventh step: Proliferation and toxicity assay using CCK-8.6 The CCK-8 assay recommends a minimum density of 2,000 cells per well for cell viability and 5,000 cells per well for cell toxicity using a 96 well plate. A well must contain 100µL of the solution, i.e. a density of 2x104 and 5x104 cells/mL for the viability and the toxicity tests respectively. Make the calculations so that you have the desired densities. We can only use 6 mL (60 wells of 100µL each) to inoculate our cells because the wells at the ends must be occupied by PBS which will evaporate and wells 2 and 3 by the culture medium (blank). Seed the 96 well-plate according to the recommendations above. Avoid the presence of bubbles. Make sure that the medium is always homogeneous for a good distribution of the number of cells in the wells. Place the plate in the incubator for 24, 48 and 72 hours;
Cell proliferation
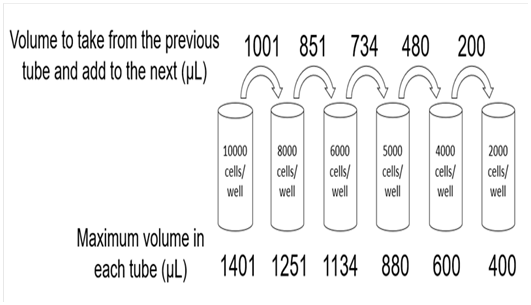
Figure 1 Protocol to Seed 06 Different Densities of Cells Per Well in Triplicate Using a 96 Well Plate.
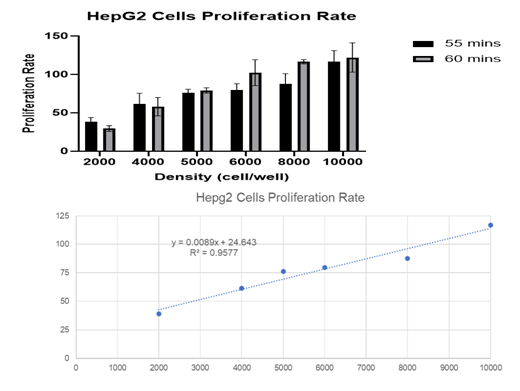
Figure 2 HepG2 cells proliferation rate. We can see that the rate of proliferation double when the cell number double. Source: ZOA BINDZI, 11/24/2020.
Cell toxicity
Note: To add plant extract in 96 well plate, follow the same protocol described in figure 1.
The equipment of a pharmacology laboratory for in-vitro studies of cytotoxic drug candidates depends if we are inside or outside the culture room. To ensure good quality results, pharmacology researcher must follow scrupulously Good Laboratory Practices (GLP) for in-vitro studies.7 Any experiment to be reliable must be repeated three times and researcher should avoid contamination by foreign bodies (bacteria, viruses, fungi, etc.), responsible for losses, especially on the financial level, because pharmacological research is quite expensive particularly in developing countries.
We want to thank The Gannan Medical University for the experience acquired in their pharmacology lab.
The author declares no competing interest.

©2021 Zoa. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.