eISSN: 2379-6367


Research Article Volume 10 Issue 3
Department of Chemical Thermodynamics, Institute of Physical Chemistry “Ilie Murgulescu” Romania
Correspondence: Ana Neacsu, Department of Chemical Thermodynamics, Institute of Physical Chemistry “Ilie Murgulescu” 202 Splaiul Independentei, 060021, Bucharest, Romania, Tel +40721249280
Received: June 17, 2022 | Published: June 24, 2022
Citation: Neacsu A, Gheorghe D. Comparative EPR study of the radical species formed during the radiolysis in polycrystalline solid state of monohydrated (L-ASN. H2O) and deuterated asparagine (L-ASN. D2O). Pharm Pharmacol Int J. 2022;10(3):106-113. DOI: 10.15406/ppij.2022.10.00371
L-asparagine monohydrate (L-Asn·H2O) and L-asparagine deuterated (L-Asn·D2O), gamma irradiated were investigated in polycrystalline state, by means of the EPR spectroscopy. The recorded spectra of L-Asn·H2O are due to the overlapping radicals spectra, having the structures R1, R2 and R3 in various ratios. The water participation at the radiolysis mechanism via the H and OH radicals formed by ionizing radiations was highlighted. The kinetic study was performed at temperatures beyond and above dehydration temperature. The high value of the preexponential factor and the positive one of the activation entropy proves that the bonds of the transition complex involving the radicals are weaker after dehydration, the rate of the radical’s disappearance reaction being fast. For L-Asn·D2O, a different behaviour was found, the recorded spectra are different from L-Asn·H2O, due to the increasing energy of the deuterium bonds. The radicalic entities ratio depends on crystalisation time after dissolution in D2O.
Keywords: monohydrated and deuterated asparagine, EPR spectroscopy, paramagnetic centers, radiolysis mechanism, kinetic study
Asparagine is one of the 20 most common natural amino acids in living organism, stable both in monohydrated and anhydrous form and among of the eleven nonessential amino acids which the body can synthesize for itself.1 Asparagine plays an important role in the metabolic control of some cell functions in nerve and brain tissue and is also used in plants as nitrogen reserve source, besides this being part of different drug and food.2 Asparagine is necessary for functioning of the brain and plays an important role in formation and functioning of proteins.3
Ionizing radiations produce indirect effects on biological environments, this being explained by their high water content (» 85%), radiations split water molecules into radicals that act on biological environment molecules. In this context it should be noted that further reactions involving these radicals and those molecules depend on a number of factors such as: their chemical structure, spatial arrangement of atoms, the presence or absence of oxygen etc. It must be specified that irradiation of biological activity compounds produces chemical modifications with consequences that are not present in the case of other substances irradiation. These modifications can cause finally alteration of the functions that living cells accomplish in tissue occurring the so-called radiations biological effect.
Free radicals play an important role in living organisms.4,5 These species are paramagnetic and the mostly used method for detecting free radicals is electron paramagnetic resonance (EPR) technique,6-8 a commonly used method for determining the effect of radiation on amino acids,9 and one of the most direct and powerful method for detection, quantification and identification of free radicals and other species with unpaired electrons.10 Literature data reveals that L-asparagine is a potential material for EPR dosimetry applications, especially for treatments employing dose rate variations, as in the intensity modulated radiotherapy.11
Asparagine it is an interesting compound having a complex structure to be subjected to a kinetic study in order to reveal the radicalic species resulted through irradiation, in both monohydrated and deuterated forms. The hydrated form crystallizes in structures exhibiting a complex network of hydrogen bonds among asparagines molecules and between asparagines and water molecules.12 The analysis of the EPR spectra of (L-Asn·H2O) indicated the presence of three radicals, stable at room temperature, one of them resulted from expulsion of a hydrogen atom from the amidic group, being preponderent, and the other two radicals resulted from decarboxylation and deamination processes. In L-Asn∙D2O, the radicalic entities ratio depends on crystalisation time after dissolution in D2O.
Materials
Comercially available polycrystalline samples of L-asparagine monohydrated (L-Asn·H2O) (Merck, purity ≥99.0%) were used. L-Asn∙D2O was obtained after dissolution of asparagine dehydrated in D2O and then recrystallized.
Methods
Irradiation
The studied compounds were irradiated at room temperature, with gamma radiation, using a 137Cs source at a dose rate of 4.102 Gy/h. The samples were white fine crystallite powders prior to irradiation. No modification of the appearance of samples was observed after irradiation.
Electron Paramagnetic Resonance
The EPR spectra of irradiated samples were recorded by means of an ART 6 instrument (Institute of Nuclear Physics-Bucharest), which operates in the X band, with a high frequency modulation of 100 kHz. The g factors were determined using the Mn2+ ion in CaO matrix, as a standard.
Complex spectra, having six main components well resolved are obtained for L-Asn·H2O, irradiated at room temperature, in solid polycrystalline state (Figure 1).
The spectrum from Figure 1 has a g factor of 1.9998 and a total width ΔHpp= 8.05 mT.
The evolution of radical concentration during irradiation of L-Asn∙H2O is shown in Figure 2 (dose rate 4.2∙102 Gy/h), representing the main signal intensity (arbitrary units) versus irradiation time.
From Figure 2, is ascertained that the signal intensity increases linearly with the irradiation dose, up to 2∙103 Gy, then slowly, tending to a constant value at higher doses. This behavior is similar for amino acids13 and is a proof that the radiolytic process involves not only the formation but also the disappearance of radicals, under the action of gamma radiation. When a constant concentration of radicals is reached, this means that the rates of the two processes become equal.
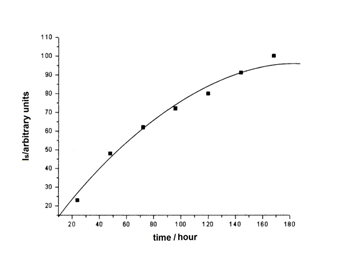
Figure 2 Variation of the ESR signal intensity, Is (arbitrary units) of L-Asn∙H2O irradiated at room temperature, versus the integral dose (dose rate 4.2∙102 Gy/h).
Figure 3 presents the EPR spectrum of L-Asn∙H2O (4∙103 Gy irradiated) sample (a) to be compared with the spectrum of the same sample after heating 4 minutes at 95°C (b).
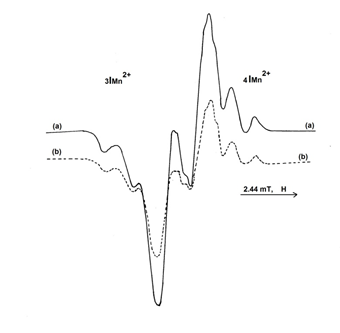
Figure 3 EPR spectrum of L-Asn∙H2O 4∙103 Gy iradiated sample (a); EPR spectrum of the same sample after heating 4 minutes at 95°C (b).
From Figure 3 it can be observed that after heating the irradiated sample, there is no modification of the spectrum structure. This behavior was met in the case of all irradiated and heated samples, during the thermal annealing study of the radicals. After heating the irradiated samples, there is no modification in the shape of the EPR spectra, this being an argument that through L-Asn∙H2O irradiation at room temperature, only one radicalic entity is formed. The EPR spectrum from Figure 1 contains six large components, the central dublet being more intense than the four lateral comoponents.
Generally, the radicals formed after amino acids irradiation in polycrystalline form, were identified only at very low temperatures (77 K). Increasing the temperature, from 77 K, the radical formed turns in to another radical via inter or intramolecular processes.14-16 The molecular structure of monohydrated asparagine is presented in Scheme 1.17,18
The side chains of asparagine molecules are bonded one to another through the hidrogen bonds of the water molecules. Each amino acid molecule performs seven hidrogen bonds, five of them are from the protons atached to the (N1 and N2) nitrogen atoms, and another two protons bonded by the oxygen atom of the water molecule. The five bonds NH...O of each molecule are performed with five neighbour molecules of amino acid, resulting a tridimensional network. The oxygen of the water molecule acts as a donor of two hydrogen bridges and acceptor for one from the nitrogen atom N1. Each water molecule through the hydrogen atoms forms two hydrogen bonds with the oxygen atoms of two different molecules of asparagine. There is no information regarding the existence of the hydrogen intramolecular bonds, which confirms the fact that the asparagine molecules in crystalline state is an open chain.2 In conclusion, the L-Asn∙H2O is stabilised through a complex lattice of seven hidrogen bonds, involving all the hydrogen atoms bonded to the nitrogen and oxygen, as presented in Scheme 1.
The EPR spectrum of the L-Asn∙H2O polycrystalline samples irradiated at room temperature, was analysed by Close et al.,15 and concluded that the main radicalic species is R1:
which do not results through deamination, but through an extraction process of a hydrogen atom from the methylen group of the amino acid chain. The central doublet recorded in the spectrum presented in Figure 1, is attributed to this radical. The two intense central components having the hyperfine splitting of 1.8 mT, are due to the odd electron interaction with the α-hydrogen atom. On the central doublet (Figure 3) it can be observed the hyperfine splittings of 0.75 mT due to the odd electron interaction with the nitrogen atom of the amino group (NH3). In order to identify the radicalic species, formed on L-Asn∙H2O irradiation, supplementary experiments were performed. A sample of L-Asn∙H2O was dehydrated in an oven at 90°C for 1 hour. The same sample was rehydrated by dissolution in water and then recrystallized. In Figure 4 are shown the EPR spectra of L-Asn∙H2O from Merck (4a) and a rehydrated sample (4b). It can be observed that the EPR spectra have the same structure.
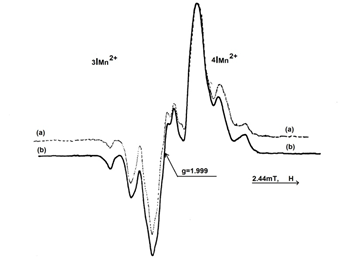
Figure 4 EPR spectrum of L-Asn∙H2O (Merck), 4∙103 Gy iradiated sample (a); EPR spectrum of the L-Asn irradiated sample after rehydration (b).
A significant experiment that proved the formation of another radical entity was the irradiation of a L-Asn∙D2O sample. For this purpose, L-Asn dehydrated was recrystallized after dissolution in D2O. In Figure 5 are shown the overlapping EPR spectra of the irradiated L-Asn∙H2O (Merck) (5a) and L-Asn∙D2O (5b).
The EPR spectrum (5b) of the recrystallised D2O sample reveals the disappearance of the two lateral components and on the central doublet it can be found the hyperfine splittings of a triplet, marked with arrows, due to the nitrogen atom interaction. The obtained result is an argument to assign the electron interaction with the nitrogen atom from amino group of the R1 radical. The hyperfine coupling with the nitrogen atom was estimated by Close15 to be 0.55 mT, on the irradiated monocrystal of L-Asn∙H2O. The absence of the odd electron interaction with the β hydrogen atom and the existence of a weak interaction with the nitrogen atom was previous proved by Close15 by EPR and ENDOR techniques. The absence in the irradiated samples of L-Asn∙D2O of the two side chain components reveal that they belong to another paramagnetic centre; its structure will be presented afterwards.
In order to highlight the thermal stability of the radicalic species resulted after L-Asn∙H2O irradiation, the reaction isochronous was plotted. For this purpose, a sample irradiated with a dose of 5.104 Gy was gradually heated for 5 min in stepwise (each step=10°C) from room temperature up to the one of the radicals disappearance. After each isothermal heating time, the EPR spectrum at room temperature was recorded. In Figure 6 is plotted the central signal intensity versus heating temperature.
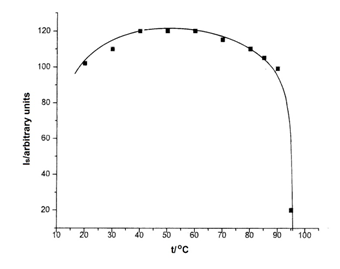
Figure 6 Isochronous variation of the EPR signal intensity (arbitrary units) versus heating temperature of a L-Asn∙H2O (Merck) sample, 5∙104 Gy irradiated.
From Figure 6 is observed a slight increase of the signal intensity up to 60°C, followed by a decrease starting from 80°C. The total dissapearance of radicals at the working condition mentioned above take place at 95°C.
The thermal dissapearance kinetics of the radicals was performed at the temperatures mentioned on reaction isochronous, in the range between 70°C-95°C. At 60°C, the signal intensity decrease was insignificant, only of 5% after isothermal heating for four hours.
The dehydration temperature of L-Asn∙H2O was 82°C, established by DSC method.19 The kinetic study was performed at temperatures below and above dehydration temperature. The signal intensities isothermally recorded at temperatures below the dehydration one, presents first a slight increase accompanied simultaneously by the central line decrease which delimitates the intense doublet (Figure 7). The detected growth do not alter the spectrum structure.
The recorded spectra present the central line as a singlet, poorly resolved, of low intensity. The singlet existence could be assigned probably to a peroxo radical resulted from R1–atmospheric oxygen reaction. During the isothermal heating, it decomposes and partially restore R1 radical.20 Figure 8 presents the variation of the signal intensities versus isothermal heating time, at the temperatures of 70°, 75°, 80°C (a) and 85°, 90°, 95°C (b), above dehydration temperature.
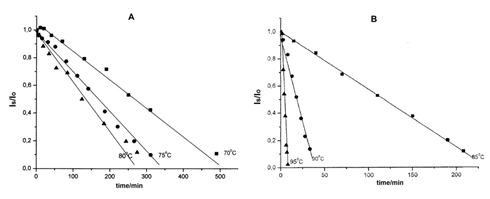
Figure 8 A- Isothermal variation of the EPR signal intensity (arbitrary units) versus time of heating at the temperatures: 70°, 75°, 80°C; B- Isothermal variation of the EPR signal intensity (arbitrary units) versus time of heating at the temperatures: 85°, 90°, 95°C.
In order to study the kinetics of the thermal disappearance of radicals, we tried to fit our data in integral equations, used in chemical kinetics. It was observed that linear plots are obtained, for all six temperatures only when the ratio [Is/Io] is expressed as a function of the time of isothermal heating (Is denotes the intensity of the signal at the moment t, while Io is the initial intensity). This proves that the thermal disappearance of the radicals obeys the kinetics of zero order.
The values of the rate constants, for each heating temperature, were calculated from the slopes of the straight lines (Table 1).
|
t, °C |
T, K |
1/T, K-1 |
k |
lgk+5 |
Ea |
A |
ΔS*, J mol K-1 |
|
70 |
343.15 |
2.915 |
3.14.10-5 |
0.496 |
|||
|
75 |
348.15 |
2.873 |
4.99.10-5 |
0.698 |
66.504 |
4.43.105 |
-29.23 |
|
80 |
353.15 |
2.832 |
6.085.10-5 |
0.784 |
|||
|
85 |
358.15 |
2.793 |
7.006.10-5 |
0.845 |
|||
|
90 |
363.15 |
2.755 |
45.10-5 |
1.653 |
353.783 |
3.21.1047 |
9.73 |
|
95 |
368.15 |
2.717 |
178.10-5 |
2.25 |
|
|
|
Table 1 The kinetic parameters values for the thermal annealing of the radicals formed on L-Asn∙H2O irradiation
Activation energies and pre-exponential factors were calculated from the Arrhenius graphic plots (Figure 9A and 9B).
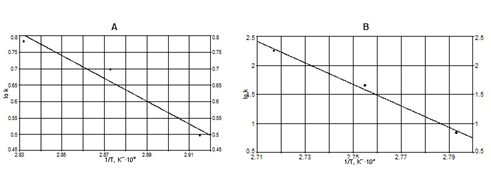
Figure 9 Arrhenius plot ln k versus 1/T beyond dehydration temperature (A) and above dehydration temperature (82°C) (B).
The activation entropies ΔSo* were calculated from the values of the pre-exponential factors, with the following equation:
(1)
where h = 6.625.10-34 J s; kB = 1.38.10-23 J K-1; R = 8.31 J mol K-1; m=0
Beyond dehydration temperature, the standard activation entropy is negative DS*= -29.23 J mol K-1 and above the dehydration temperature is positive DS*= 9.73 J mol K-1.
The slow rate of the radical’s disappearance reaction beyond dehydration temperature, is in agreement both to the low value of the pre-exponential factor, but also with the high negative value of the activation entropy. Indeed, in this case, the process of the radical’s recombination is much hindered by the intermolecular forces performed by the hydrogen bridges of the water molecules. The kinetic study performed at temperatures above the dehydration, lead to opposite results. The high value of the pre-exponential factor and the positive one of the activation entropy proves that the bonds of the transition complex involving the radicals are weaker after dehydration, the rate of the radical’s disappearance reaction being fast. As it is known, the reactions of zero order are characterized by the fact that the reaction rate does not depends on reactants concentration. The presence of seven hydrogen intermolecular bonds per molecule explains why the melting temperature of this amino acid is high, of about 225°C.21 The previous kinetic study showed that the radicals disappearance take place fast at 95°C, below the melting temperature. This experimental finding is an argument that through L-Asn∙H2O irradiation, the multiple hydrogen bonds of the radicals with the sourounded molecules are affected, this being the main factor of stabilizing the radicals in the crystalline lattice.22 Based on this hypotesis are the similar experimental findings from literature,23,24 performed on inorganic molecules, when it was found that the thermal annealing of the radicals take place at temperatures closer to the melting of irradiated substance. In the case of this amino acid, the behavior is opposite, this being a proof that the decisive factor in the radicals dissapearance is the hydrogen bonds breaking. Analysing the Figures 9 it is found that the radicals dissapearance at temperatures above dehydration one take place at high rate. This behavior demonstrates that the radicals are also stabilised through the hydrogen bonds of the water molecules. By splitting these bond, the amino acid dehydration takes place, the radicals become destabilised, their mobility increases and their ability to recombine accordingly.25,26
Another important factor to consider is the existence of a water molecule performing hidrogen bonds with each amino acid molecule, through a complex lattice presented in Scheme 1, this being an argument of water participation at radiolysis mechanism of amino acid, via the H and OH radicals, also formed by ionising radiation.
The EPR spectra of the irradiated samples isothermally heated of L-Asn∙H2O do not modify its structure, this finding being an argument to consider the formation of a single paramagnetic center. An interesting behavior was observed for the L-Asn∙H2O dehydrated and recrystallised from D2O. Depending on the crystalisation time of amino acid in D2O, the irradiated samples spectra were different. In Figure 10 are plotted three recorded spectra of irradiated L-Asn∙D2O, after three times of crystalisation.

Figure 10 The change in the EPR spectra structure of the irradiated samples of L-Asn∙D2O after different times of recrystalisation.
The analysis of the EPR spectra from Figure 10, leads to the following conclusions:
The isothermal heating of the irradiated samples of L-Asn∙D2O was performed. As opposed to L-Asn∙H2O, on deuterated samples, it was observed the spectral structure modification, during the thermal annealing process. This finding is a demonstration that the recorded spectra of the deuterated samples result from the overlapping ones of two paramagnetic centres.
An irradiated sample having the structure 10 B, after heating up to 95°C, presents a decrease of the spectral lines, being different from L-Asn∙H2O. After 8 minutes isothermal heating, the central doublet intensity decreased about 80%, while the extreme components decreased only about 43% (Figure 11).
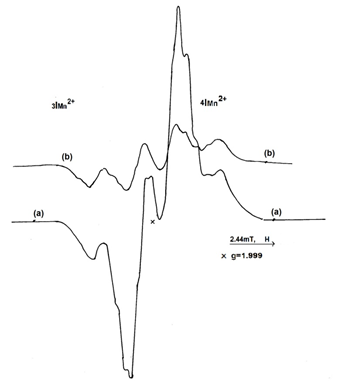
Figure 11 The EPR spectrum of L-Asn∙D2O, 2∙104 Gy irradiated sample (a); The EPR spectrum of L-Asn∙D2O, 2∙104 Gy irradiated sample after 8 minutes heating at 95°C (b).
This result is an argument that the obtained spectrum after 8 minutes heating, belongs to a radicalic entity having high thermal resistance. The intensity of the four components from Figure 11(a) has the ratio 1:2:2:1. Both the shape of the spectrum and the lines intensities ratio are similar to the spectrum presented in Figure 10C, recorded from a L-Asn∙D2O sample at room temperature.
The spectrum from Figure 11 is due to the R2 radical, obtained through decarboxylation, having the structure:
The assignment of this radical to the recorded spectrum is due to the following considerations:
From the recorded experimental data presented above results that the EPR spectra of the room temperature irradiated samples of L-Asn∙H2O, are due to the overlapping radicals spectra having the structures R1, R2 and R3, formed in various ratios. In the irradiated samples of L-Asn∙H2O, R1 is the prevalent radical, in the case of L-Asn∙D2O, the entities ratio R1 and R2 varies depending on the time of crystalisation after dissolving in D2O. In order to justify the preponderance of R1 radical in the irradiated samples of L-Asn∙H2O, in Figure 12a was plotted the variation of the lateral component intensity Ia and the central component Ib of the EPR spectrum, versus isothermal heating time at 85°C (Figure 12A).
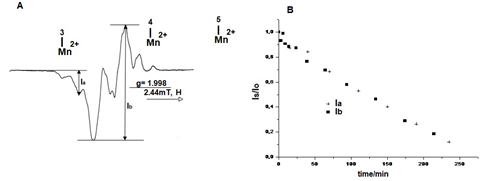
Figure 12 Variation of the signal intensities Ia(+) şi Ib(■)versus isothermal heating time (A), 85°C (B) of L-Asn∙H2O irradiated sample.
The plot from Figure 12 shows that both components of the spectrum present an identical linear decrease, having the same slope.
Both R1 and R2 radicals dissapear at this temperature but their thermal stability being different, their slopes should be different. The obtained result is in contradiction with the assessment of the spectral lines to two different radicalic species.The reason why it is not observed a different variation of the lines although they belong to different radicals, is due to the high concentration of R1 radicals versus R2 and to the deciding factor of both radicals dissapearance which is the same, namely, breaking of their hidrogen bonds.
The isothermal dissapearance at 90°C of the radicals belonging to an irradiated sample of L-Asn∙D2O presenting the spectrum from Figure 10A, was performed to compare with the isothermal dissapearance, at the same temperature, of L-Asn∙H2O, being found a different behaviour.
The total dissapearance of the radicals in L-Asn∙D2O occured in a longer time, after 175 minutes versus 30 minutes in L-Asn∙H2O. Besides this, the zero ordin kinetics is no longer observed. This behaviour could be attributed on the increasing energy of the deuterium bonds in the L-Asn∙D2O samples. An important contribution to this process is also the decrease of the vapour pressure of D2O compared to H2O, due to the increase of the intermolecular interactions, of the coulomb attraction forces between electronic layer and positive nuclei respectively.
In Figure 13(a) is plotted the spectrum of L-Asn∙D2O irradiated sample, compared to the spectrum of the same sample after 9 minutes isothermal heating at 90°C (Figure 13b). It can be observed a strong decrease of the central lines accompanied by a highlighting of the hyperfine splittings. This process is accentuated with the increase of the isothermal heating time, finally reaching a stationary state. For exemplification, in Figure 14 is plotted the spectrum of the same sample after 85 minutes heating at 90°C.
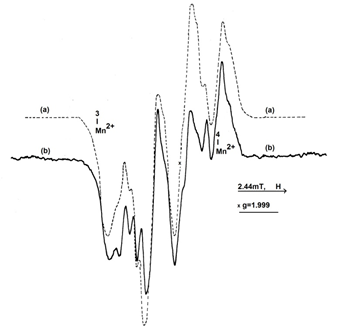
Figure 13 The EPR spectrum of L-Asn∙D2O, 4∙103 Gy irradiated (a); The EPR spectrum of L-Asn∙D2O, 4∙103 Gy irradiated, after 9 minutes isothermal heating at 90°C (b).
To prove that EPR spectrum of the irradiated sample of L-Asn∙D2O is the result of the overllaping spectra of two radicalic entities, the variation versus isothermal heating time at 95°C of the I1 intensity of the lateral and central (I2) component, was investigated (Figure 15a).
From Figure 15B is observed that the intensity of the central line I2 decreases more than the lateral I1. Both components present a linear decrease in time and finally tend to a plateau.

Figure 15 Variation of the signal intensities Is1 şi Is2 (A) versus isothermal heating time at 95°C (B) of an irradiated sample of L-Asn∙D2O.
The plot for L-Asn∙D2O from Figure 15B is similar with the plot for L-Asn∙H2O from Figure 12b, but the results are different. From the results obtained above, the following conclusions can be ascertained:
The kinetic study of the radicals thermal dissapearance was performed on L-Asn∙D2O 104 Gy irradiated sample, having the EPR spectrum from Figure 13a. For this purpose, the isothermal variation at 90°C of the I2 and I1 components intensities versus heating time was plotted. Using the relative values of the ratio I2/Io2 and I1/Io1 we tried to fit our data in integral equations, used in chemical kinetics. For the radicalic species corresponding to the I2 intensity, plotting versus isothermal heating at 90°C, a straight line was obtained, demonstrating that the radicals disappearance obeys the kinetics of fractional order 1.5 (Figure 16A).
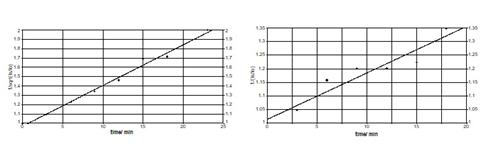
Figure 16 Variation of the ratio (I2/Io)-1/2 versus isothermal heating time at 90°C (A);Variation of the ratio (I1/Io)-1 versus isothermal heating time at 90°C (B).
A similar kinetic study was performed for the radicalic species corresponding to the I1 intensity, plotting 1/(Is1/Io) versus isothermal heating at 90°C, a linear plot was obtained , proving that the radicals disappearance obeys a second order kinetics (Figure 16B).
From the kinetic results presented above, the following conclusions can be ascertained:
The radiolysis mechanism
The mechanism of radicals formation due to gamma irradiation in L-Asn∙H2O has been less studied. According to literature data15,16 and to the results presented above, the radiolysis mechanism was established (Scheme 2). It must be observed that like most amino acids, the two functional groups of asparagine molecules when crystallizing, perform a zwitterion structure.
Direct ionisation of L-Asn molecule leads to the radical-cation (Asn·+) , spin density is distributed mainly on the oxygen atoms of amidic and carboxylic groups.27,28 This process leads to the formation of two radical-cations: Asn(COO)·+ and Asn(CONH2)·+.
The radical cation Asn(COO)·+ is the precursor of the decarboxylated radical Asn(C·H), noted R2, identified in the EPR spectrum at 250 K and CO2 was cromatographically detected.16
The radical cation (Asn·-) is the precursor of the (Asn·-) radical, noted R3, obtained after deamidation. This radical formation was also established from the EPR spectra, recorded both at room temperature and above.16 The radical-anion Asn(CONH2)·+, resulted by capturing the electron from the carboxyl group oxygen, was observed in the EPR spectra as a doublet, at 77 K, only. There is no proof that this radicalic entity dissapears after deamination, as in the case of other amino acids irradiation.29 Strzelczak et al.,16 considers that the radical-anion AsnC·H2, being unstable, expells a hydrogen atom from the amino group. This atom being very reactive in turn extracts a hydrogen atom from the methylene group of the main chain of the amino acid, generating the R1 radical, stable at room temperature, the kinetics of its thermal dissapearance being the object of this study.Simultaneously with the radical-anion formation, the expelled hydrogen atom extracts a H atom from the same molecule, resulting R1 radical. In contrast to L-Asn∙H2O, in the case of other amino acids radiolysis, it was found that the formation of the radical resulted from hydrogen extraction from parent molecule is produced by the decarboxylated and deaminated radicals, previously formed.29 As it is known, gamma radiations, unlike the photochemical ones, are not selectively absorbed, in the case of L-Asn∙H2O, by the amino acid molecule only, they acts randomly upon all molecules which compose the irradiated substance, therefore the radyolisis mechanism of L-Asn∙H2O must include the Asn·+ and radicals participation, resulted from the water radiolysis.
Consequently, the H atom which generates the R1 radical it does not come from L-Asn molecule only, but also from the water molecules or maybe only from water molecules.Considering the involvment of the resulted radicals from the water, the radiolysis mechanism of L-Asn∙H2O become more complex.
It should be specified that the resulted electron from the main radiolitic process, does not come exclusively from the carboxyl group of the amino acid, it is formed also through direct ionisation of the water molecules. The same electron after loosing the kinetic energy could be captured by the Asn molecule in order to form the radical-anion but, also with a higher probability by the positive ion from the water molecule, due to columbian attraction.
Two parallel EPR studies on L-Asn∙H2O and L-Asn∙D2O were performed, in order to highlight the water participation at the radiolysis mechanism. The mechanism of radiolysis was established. Three paramagnetic centers, R1, R2, and R3 are formed after L-Asn∙H2O irradiation at room temperature; R1 being the prevalent one; in the case of L-Asn∙D2O, the entities ratio R1 and R2 varies depending on the time of crystalisation after dissolving in D2O. The kinetics of the thermal disappearance of radicals for the L-Asn∙H2O and L-Asn∙D2O was performed. The kinetics of zero order was obtained for L-Asn∙H2O and the parameters values for the thermal annealing of the radicals were calculated. Through L-Asn∙H2O irradiation, the multiple hydrogen bonds of the radicals with the sourounded molecules are affected. L-Asn∙D2O radicals disappearance obeys the kinetics of fractional order 1.5. The kinetic study revealed that R1 and R2 radicals formed in L-Asn∙D2O have higher thermal resistance than those formed in L-Asn∙H2O. The isothermal dissapearance, at the same temperature, 90°C, for both L-Asn∙D2O and L-Asn∙H2O was performed, and a different behaviour was found. The zero ordin kinetics was no longer observed for L-Asn∙D2O. This behaviour is due to the increasing energy of the deuterium bonds in the L-Asn∙D2O samples.
The authors are grateful to University of Bucharest, Faculty of Chemistry for technical support and for the use of radiation sources and to prof. Dr. Mihail Contineanu for critical review of the manuscript.
Authors declare that there is no conflict of interest.

©2022 Neacsu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.