eISSN: 2379-6367


Short Communication Volume 12 Issue 4
1Clinical Pharmacokinetics Center, University of Sao Paulo–Sao Paulo/SP, Brazil
2Plastic Surgery-Burn Division, Surgery Department of Medical School, University of Sao Paulo/SP, Brazil
3Central Laboratory Division and Medical Investigation Laboratory (LIM-03) – Medical School, University of Sao Paulo (HCFMUSP)
Correspondence: Silvia R C J Santos, Clinical Pharmacokinetics Center, University of Sao Paulo - Sao Paulo/SP, Brazil, Tel 55 11 953578930
Received: July 29, 2024 | Published: August 19, 2024
Citation: Santos SRCJ, Kupa LVK, Santos MJ, et al. Biomarkers of septic shock to predict hospital mortality in major burns undergoing antimicrobial therapy during the systemic inflammatory response syndrome. Pharm Pharmacol Int J. 2024;12(4):138-142. DOI: 10.15406/ppij.2024.12.00444
Introduction: Septic shock is one of the leading causes of death among critically ill patients in the Intensive Care Unit (ICU), including severely major burned patients. Rapid diagnosis and early initiation of effective antimicrobial therapy are the main challenges. The role of leukocytes as the neutrophil/lymphocyte ratio (NLR) and various systemic inflammatory indices, include serum levels of interleukin-6 (IL-6), procalcitonin (PCT) and c-reactive protein (c-RP) are the informative laboratory markers in this regard respect.
Subject: Monitoring of serum levels of interleukin (IL6), procalcitonin (PCT) and C-reactive protein (C-RP), including among leukocytes the index neutrophil/lymphocyte ratio (NLR), proposed previously, investigated at the first septic shock of major burns undergoing meropenem therapy recommended in hospital, during the systemic inflammatory response syndrome (SIRS), after ICU admission. Fundamental aim of study was to investigate the relationship, and contribution of these biomarkers at the early versus late stage of septic shock, as well as to determine the diagnostic performance of them in major burn patients that could impact outcome.
Methods: In this prospective open label study, a total of 30 major septic burn patients with renal function preserved, augmented or with acute kidney injury (AKI) was enrolled at the first septic shock after accident and ICU admission, based on systemic inflammatory response syndrome (SIRS) criteria. All patients had been selected from the ICU of Plastic Surgery and Burn Unit of tertiary public hospital of Medical School, University of Sao Paulo, SP, Brazil. Laboratory data with known clinicopathological parameters were recorded. Serum levels of IL6, PCT, c-RP and NLR from the blood count were evaluated. Inflammatory biomarkers such as c-RP, PCT and IL6 in serum were performed on the COBAS 8000 series (c-RP) or COBAS E411 series analyzer for PCT, IL6 (Roche, registered trademark), neutrophil to lymphocyte ratio (blood count) was measured using a Hematology Analyzer (SYSMEX brand). Therapeutic serum measurements of combined therapy with antibiotics (ATB) were done by comparison of coverage at the earlier versus late stage of septic shock.
Results: A significant difference was found in NLR, IL6, PCT and c-RP in surviving patients (n=20) comparing data obtained in early stage versus late-stage SIRS (p<0.05) in major burns with positive bacteriological cultures. On the other hand, there was no significant difference between the NLR and IL6 periods, which occurred when comparing data in the early versus late stage of SIRS in non-survival patients, who died between 7 and 10 days of antimicrobial therapy. There was an increasing trend in serum levels of NLR and IL6, PCT and c-RP in large burns, dependent on the timing of septic shock stages, with diagnostic value as early appearance of biomarkers. On the other hand, we recorded that the association of NLR and IL6 produces better diagnostic value in predicting ICU mortality than PCT or c-RP.
Conclusion: It was shown in this pilot study that elevated NLR and increasing serum levels of IL6, PCT, c-RP occurred during SIRS in septic patients’ major burns. So, the combined use of these biomarkers may play a potential role in the early diagnosis of septic shock for adequate initial therapy of these ICU patients. Combined biomarkers (NLR-IL6) can further predict ICU mortality of septic patients with acute kidney injury occurring during SIRS. Finally, a prospective multicenter study in a large cohort can be performed to confirm the data obtained in this investigation.
Keywords: NLR, IL6, PCT, c-RP during SIRS, combined NLR-IL6 to predict mortality in acute kidney injury in major septic burns, meropenem therapy
AKI, acute kidney injury; ATB, antibiotic; C-RP, C-reactive protein; CLSI, clinical laboratory standards institute; CVVHD-F, continuous venovenous haemodialysis-filtration; FDA, food and drug administration; GSA, global sepsis alliance; ICU, intensive care unit; IL6, Interleukin-6; MDR, multidrug resistance; MIC, minimum inhibitory concentration; MV, mechanical ventilation; NLR, neutrophils to lymphocytes ratio; PCT, procalcitonin; PD, pharmacodynamics; PK, pharmacokinetics; PK/PD, pharmacokinetics/pharmacodynamics; PNM, Pneumonia; PTA, probability of target attainment; RFA, renal function augmented; RFP, renal function preserved; SAPS3, simplified acute physiology score 3; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SSC, surviving sepsis campaign; SIRS, systemic inflammatory response syndrome; TBSA, total burn surface area; TDM, therapeutic drug monitoring; WHO, world health organization
Septic shock is a potentially fatal organ dysfunction caused by a dysregulated host response to infection, is a preventable event. However, the clinical outcome in most high-risk cases is death in patients with nosocomial bacterial infections associated with multiple comorbidities including metabolic syndrome, and viral infections, most recently SARS-CoV-2.1,2 Around 60 million cases diagnosed annually around the world, at least 12 million patients die in ICU, mainly in underdeveloped countries. In addition, data of a large international prospective study show that more than 80% of patients admitted to the ICU receive antibiotics and 50% of them with a poor prognosis deceased.3 However, the incidence of infections and associated mortality in seriously ill patients admitted to ICUs has not improved in the last 10 years.4 This fact indicates that it may be possible to improve septic patient care and clinical outcomes based on serum levels of new inflammatory biomarkers currently under investigation, such as interleukin-6 (IL6) and procalcitonin (PCT) combined with the well-known c- reactive protein (c-RP) serum levels, considered an excellent predictive index of septic shock in these patients.5–10
In addition, concerning renal dysfunction of high incidence in critically ill patients that occurs in ICU during the systemic inflammatory response syndrome (SIRS), it was found a quite interesting contribution reported by Shimazui et al.,8 based on IL-6 serum levels on ICU admission and subsequent outcomes in septic patients with acute kidney injury. It is well known that the exacerbated inflammatory response is considered one of the key elements of acute kidney injury (AKI). Then, interleukin-6 (IL-6) is an inflammatory cytokine that plays important roles in the inflammatory response and may be useful for predicting the clinical outcomes in patients with AKI.9 More recently, a very interesting data related to combined inflammatory biomarkers interleukin-6 serum levels and neutrophil-to-lymphocyte ratio (NLR) based on hemograms were proposed in predicting 28-day ICU mortality in critically ill patients with sepsis was reported by Liu et al.10 Thus, this population requires an immediate change in the behavior of the clinical team and continuous monitoring of these patients undergoing intensive care through continuous hemodynamic, respiratory, renal, and infectious surveillance.
In a monitoring program of antimicrobial therapy for several infections based on serum levels of beta-lactams in patients admitted to the ICU, it was reported that 73% of patients did not reach the therapeutic target against susceptible strains of Gram-negative bacteria. This fact reinforces that inflammatory biomarkers IL6, PCT, c-RP and NLR monitoring, as predicting indexes of septic shock, combined with serum levels of antibiotics is essential to assess changes in pharmacokinetics that impact the coverage of ATBs during the antimicrobial therapy prescribed to those patients. Therefore, new therapeutic strategies have been proposed for the most prescribed agents against Gram-negative nosocomial pathogens as meropenem and piperacillin-tazobactam related to the dose regimen and duration of infusion monitored by pharmacokinetics-pharmacodynamic approach.11 Finally, based on several studies going on, the planned implementation of economic monitoring of inflammatory biomarkers combined with serum levels of the prescribed antibiotic will allow dose adjustment in real time, guided always by biomarker index indicating death, to reduce mortality of these septic patients in ICU.
Objective
The aim of the study was to investigate four inflammatory biomarkers based on serum levels of interleukin-6 (IL6), procalcitonin (PCT) and c-reactive protein (c-RP), combined with the neutrophil/lymphocyte ratio (NLR) index among leukocytes, in major burn patients in SIRS at the first septic shock, undergoing antibiotic therapy against Gram-negative nosocomial pathogens during systemic inflammatory response syndrome (SIRS). Fundamental aim of study was to investigate the relationship, and contribution of these biomarkers at the early versus late stage of septic shock, as well as to determine the diagnostic performance of them in major burn patients that could impact outcome.
Study design, patient eligibility
The clinical protocol was a prospective, open-label study. Ethical approval registers CAAE 07525118.3.0000.0068, Brazilian Platform was obtained by approval of the Ethical Committee of Hospital of Clinics, Medical School of University of Sao Paulo; no conflicts of interest to declare were obtained from all authors. The study was conducted from August 2018 to March 2020, and informed written consent was obtained from all legally designated patient representatives. Adult patients from the Intensive Care Burn Unit, presenting severe thermal injury and a sepsis diagnosis was according to the “American Burn Association consensus conference to define sepsis and infection in burns” in clinical evaluation and laboratorial data were eligible for inclusion.
Complete medical history, physical examination was obtained for each enrolled patient; laboratory data including biomarkers monitoring, meropenem serum levels. Microbiology of isolated strains documented in blood cultures, bronchoalveolar lavage, wound/bone, and urinary tract were considered. Susceptibility testing was done to obtain the minimum inhibitory concentration for each antimicrobial agent against each pathogen isolated in the Clinical Microbiology of central laboratory of hospital, considering CLSI data base. A total of 30 major septic burn patients with renal function preserved or with acute kidney injury (AKI), with positive cultures was enrolled at the first septic shock, at least after 72 hours of ICU admission, based on systemic inflammatory response syndrome (SIRS) criteria. All patients had been selected from the ICU of Plastic Surgery and Burn Unit of tertiary public hospital of Medical School, University of Sao Paulo, SP, Brazil. Laboratory data with known clinicopathological parameters were recorded. Inflammatory biomarkers were monitored daily in serum c-RP and NLR in blood count (hemogram), while serum levels of IL6 and PCT were measured every two days during SIRS. Meropenem was prescribed according to renal function, and cultures were collected before the antimicrobial therapy starts and during all SIRS periods. Serum levels of inflammatory biomarkers such as C-reactive protein (c-RP), procalcitonin (PCT) and interleukin-6 (IL6) and neutrophil to lymphocyte ratio (NLR) from blood count were evaluated. Then, c-RP, PCT and IL6 in serum were performed on the COBAS 8000 series (C-RP) or COBAS E411 series analyzer for PCT, IL-6 (Roche, registered trademark), while NLR (blood count) was measured using a Hematology Analyzer (SYSMEX brand).
All patients received meropenem therapy against Gram-negative nosocomial pathogens, recommended in our hospital after ICU admission. Serum measurements of chosen antibiotic (ATB) prescribed to these patients were done by comparison of coverage at the earlier versus late stage of septic shock for each patient. Meropenem in serum was quantified by liquid chromatography in the Center of Clinical Pharmacokinetics.12 All data of the tests carried out in the hospital's Central Laboratory, including cultures were sent to the ICU via the network.
Statistical analysis
Individual and population data: actual statistics of this study conducted on 30 major burn patients after ICU admission, at the first septic shock during SIRS, was done based on the use of software’s described as follows: OFFICE 365, version 2208 (Excel); GraphPAD Instat-GraphPad Prism version 9.1.14 and version 10. Fisher's exact test, Mann Whitney and Wilcoxon tests applied to unpaired and paired data were applied to results obtained from the investigated patients, significance of p<0.05 was considered.
Demographic, clinical and laboratorial data
Demographic characteristics of major burn patients’ population included in the study were: 21M/9F, 47 (39-67) yrs., 69 (63-75) kg, ideal body weight, expressed by medians (IQR). Admission data of burn patients in ICU of hospital after the accident were: simplified acute physiology score-3 at ICU admission (SAPS3) of 59 (55-68), total burn surface area of 39 (25-67) %, thermal/electrical injury (27/3), inhalation injury occurred in 25 patients requiring mechanical ventilation, and vasopressor requirements occurred in 22 patients. Thirty major burn patients enrolled in the protocol were undergoing therapy of septic shock with meropenem. Patients were distributed in two groups based on clinical outcome, survival patients (n=20), and non-survival patients (n=10). All patients were investigated in two periods considering the inflammatory biomarker measurements and therapeutic drug monitoring (TDM) of meropenem in serum at TDM1-earlier stage versus TDM2-late stage of septic shock. Twenty survival patients at the earlier stage of septic shock presented renal function preserved or augmented by vasopressors (n=19), or yet acute kidney injury (n=1). Inflammatory biomarkers measurements, and meropenem serum levels for coverage purposes were investigated at TDM1-early stage. In addition, at the TDM2-late stage of septic shock, inflammatory biomarkers and meropenem serum levels were measured again for these patients.
Then, at the first week of meropenem therapy, majority of ICU burn patients presented systemic inflammatory response syndrome (SIRS), with or without vasopressors requirements, and therapeutic drug monitoring of meropenem was required. Meropenem therapy started with the empirical dose regimen 1g q8h by 3hrs.-infusion recommended in hospital prescribed to 19/20 burn septic patients with preserved (RFP) or augmented renal function (RFA). It is important to highlight that just one of them presented acute renal dysfunction (AKI); then, meropenem was reduced to dose regimen of 1g q24h by 3hrs.-infusion. After 48 hours of meropenem therapy started, blood was sampling at the steady-state level for therapeutic drug monitoring (TDM1) done in our laboratory by liquid chromatography.12 After 48 hours of meropenem therapy started, blood was sampling at the steady-state level for therapeutic drug monitoring (TDM1). Furthermore, in the early phase of septic shock, it was shown minimum inhibitory concentration (MIC) up to 2 mg/L from isolates from cultures; therefore, meropenem coverage was guaranteed for all patients up to MIC 4 mg/L, although no intermediate susceptibility pathogen (MIC 4 mg/L) was recorded. Therefore, negative cultures were registered between 7-12 days of meropenem therapy in surviving patients.
Another group considered was related to non-survival patients with renal function preserved or augmented by vasopressors (n=7), or yet presenting acute kidney injury (n=3) at the earlier stage of septic shock. Inflammatory biomarkers measurements, and meropenem serum levels were investigated at TDM1-early stage. At the late stage of septic shock, inflammatory biomarkers and meropenem serum levels were investigated again for these patients with acute kidney injury registered in all of them at TDM2-late stage. Despite meropenem coverage of isolated strains from cultures with MIC up to 2 mg/L, at the early phase of septic shock, after blood sampling for meropenem serum measurements at TDM2-late stage; unfortunately all these patients deceased between 7th and 10th day of meropenem therapy because of SIRS.
Inflammatory biomarkers monitoring
Several studies of inflammatory cytokines employed as biomarkers have been reported for patients with sepsis or septic shock. However, there was high variability in the data reported in most studies published in the last 10 years, and no investigation in burn patients was found.4–10 In our pilot study, thirty patients were included, and four chosen inflammatory biomarkers were monitored. The protocol was designed to investigate inflammatory biomarkers in severely burned ICU patients undergoing meropenem therapy, considering the first septic shock after admission. Blood samples were collected at the set-up of septic shock for IL6 and PCT serum measurements, and every two days from the beginning to the end of meropenem therapy over a maximum period of two weeks (day 2 to day 14). Serum c-RP levels and NLR-based blood counts were performed daily as occurs routinely in ICU patients done in the hospital's central laboratory division of our hospital. Biomarker expression was investigated during meropenem therapy, and clinical outcome in ICU 30-day mortality was considered by ICU-death (non-survivors) or surviving patients. In addition, the length of stay in hospital was based by ICU death, or by ICU followed by hospital discharge. Inflammatory biomarker expression data were described in table 1, based on serum levels of IL6, PCT, c-RP and NLR (blood count) in parallel with therapeutic drug monitoring of meropenem serum levels at early stage versus late stage of SIRS, in parallel to meropenem therapy: TDM1 on the 2nd day of starting therapy versus TDM2 at the end of therapy, 10th to 12th day. The frequency of TDMs in general was once a week for patients with preserved renal function, or twice a week for patients with renal function increased by vasopressors, or with acute kidney injury to ensure the efficacy and safety of the drug for all of them.
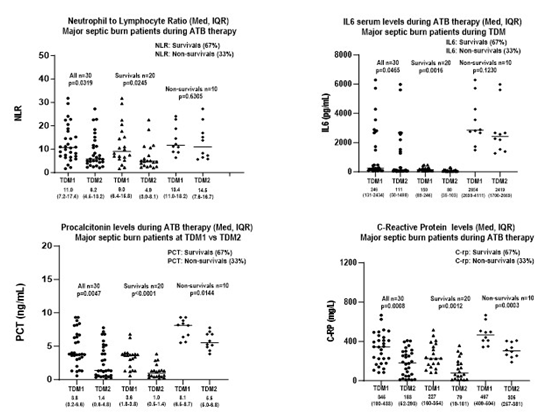
Considering septic major burn survival patients, it was shown increasing serum levels of IL6, PCT, c-RP, as well elevation on Neutrophil to Lymphocyte-Ratio (NLR) at the earlier versus late stages of SIRS. All biomarkers were measured at the same day of meropenem serum monitoring in ICU-survivor patients; expressed as medians were compared in both earlier versus late stages, Table 1, Figure 1. It was registered significant differences between both stages of SIRS investigated, since a faster decrease occurred in survival patients with major reductions on NLR, in serum levels of IL6, PCT, c-RP, registered over time for NLR (9.0 versus 4.9, p=0.0245), IL6 (150 versus 80 pg/mL, p=0.0016), PCT (3.6 versus 1.0 ng/mL, p<0.0001), c-RP (227 versus 79 mg/L, p=0.0012). On the other hand, inflammatory biomarkers were compared for non-survivors at both periods, and significant data were pointed out only for PCT and C-RP based on serum levels of PCT (8.1 versus 5.5 ng/mL, p=0.0144), c-RP (467 versus 305 mg/L, p=0.0003). In addition, in non-survivors a slower decrease occurred related to neutrophil/lymphocyte ratio, and serum levels of IL6, registered over time for NLR (13.5 versus 14.5, p=0.6305), IL6 (2854 versus 2419 pg/mL, p=0.1230). It is important to highlight that high values for all biomarkers investigated were registered at in both stages of SIRS in non-survival patients with acute kidney injury. Then, in those patients’ data were justified as a function of very high inflammatory response based on biomarkers monitored during SIRS. Significant difference was found in NLR, IL6, PCT and c-RP in surviving burn patients (n=20) comparing data obtained in early stage versus late-stage SIRS (p<0.05) in major burns with positive bacteriological culture. On the other hand, there was no significant difference between the NLR and IL6 periods, which occurred when comparing data in the early versus late stage of SIRS in non-survival patients, who died between 7 and 10 days of antimicrobial therapy. Finally, there was an increasing trend in serum levels of NLR and IL6, PCT and c-RP in major burn patients, dependent on the timing of septic shock stages, with diagnostic value as early appearance of biomarkers. In addition, increasing interleukins, including IL6 at the late stage of SIRS is an important index in predicting mortality in ICU patients as reported previously.9 According to cut-off points reported by Liu et al.,10 for combined biomarkers NLR-IL6, the limits considered were related to combined biomarkers NLR-IL6, the cut-off points were NLR = 4.94 IL6 = 117.6 pg/mL.10 On the other hand, we recorded that NLR combined to IL6 produces better diagnostic value in predicting ICU mortality than PCT or c-RP.
|
Laboratorial data (CLSI) |
Initial stage of septic shock |
Late stage of septic shock |
P |
|
Survivals n=20 |
TDM1 |
TDM2 |
|
|
Clcr (mL/min) |
88 (72-118) |
76 (64-128) |
0.7634 |
|
NLR (ratio) |
9.0 (6.4-15.8) |
4.9 (3.0-8.1) |
0.0245 |
|
IL6 (pg/ml) |
150 (89-246) |
80 (35-103) |
0.0016 |
|
PCT (ng/mL) |
3.6 (1.8-3.8) |
1 (0.5-8.1) |
<0.0001 |
|
c-RP (mg/L) |
227 (160-354) |
79 (19-181) |
0.0012 |
|
Non-survivals n=10 |
|||
|
Clcr (mL/min) |
79 (38-112) |
26 (22-33) |
0.0016 |
|
NLR (ratio) |
13.4 (11.0-18.2) |
14.5 (7.6-16.7) |
0.6305 |
|
IL6 (pg/ml) |
2854 (2690-4111) |
2419 (1700-2669) |
0.1230 |
|
PCT (ng/mL) |
8.1 (6.5-8.7) |
5.5 (5.0-6.8) |
0.0144 |
|
C~RP (mg/L) |
467 (409-504) |
305 (257-381) |
0.0003 |
|
Meropenem regimen |
Coverage up to MIC 2mg/L Coverage up to MIC 4mg/L |
P |
|
|
Survivals (renal function according Clcr) |
Susceptible strains |
Intermediate susceptibility |
|
|
TDM1: 1g q8h 3hrs.-infusion. RFA n=19/20 |
100% (19/19) |
100% (19/19) |
1.0000* |
|
TDM1: 1g q24h 3hrs.-infusion. AKI n=1/20 |
100% (1/1) |
100% (1/1) |
1.0000* |
|
TDM2: 1g q8h 3hrs.-infusion. RFP n=20/20 |
100% (20/20) |
100% (20/20) |
1.0000* |
|
Meropenem - regimen |
Coverage up to MIC 2mg/L Coverage up to MIC 4mg/L |
|||
|
Non-survivals (according Clcr) |
Susceptible strains |
Intermediate susceptibility |
||
|
TDM1: |
1g q24h 3hrs.-infusion. AKI n=3/10 |
100% (3/3) |
100% (3/3) |
1.0000* |
|
TDM2: |
1g q24h 3hrs.-infusion. AKI n=10/10 |
100% (10/10) |
100% (10/10) |
1.0000* |
Table 1 Burn septic patients undergoing meropenem therapy by extended infusion, median (IQR) Laboratorial data: Biomarkers: TDM 1 versus TDM2. Antimicrobial coverage at the first septic shock
Abbreviations: Clcr, creatinine clearance; NLR, neutrophils to lymphocytes ratio; IL6, interleukin-6; PCT, procalcitonin; c-RP, c-Reactive protein; CLSI, Clinical Laboratory Standard Institute; TDM, therapeutic drug monitoring; IQR, quartiles (25-75); ICU, Intensive care unit; MIC, minimum inhibitory concentration; RFP, renal function preserved; RFA, renal function augmented; AKI, acute renal injury.
Statistics: GraphPad Prism, v.9.1.4, Mann Whitney, *Fisher test; Kruskal-Wallis test.
It was shown in this pilot study that elevated NLR and increasing serum levels of IL6, PCT, c-RP occurred during SIRS in septic patients’ major burns. So, the combined use of these biomarkers may play a potential role in the early diagnosis of septic shock for adequate initial therapy of these ICU critically ill patients. Combined biomarkers (NLR-IL6) can further be an earlier predicting factor to reduce ICU mortality for septic burn patients with acute kidney injury occurring during SIRS. Finally, a prospective multicenter study in a large cohort can be performed to confirm the data obtained in this investigation.
We would like to thank all the staff, clinicians, and surgeons in the ICBU who took part in this study and for their support.
Author’s contributions
All authors contributed equally to this work based on their specialty. DSG contributed to the study related to ethical approvals at the hospital and the Brazilian Platform for clinical projects, data acquisition, interpretation, and critical review of the manuscript content. SS contributed to the conception and design of the study, acquisition and interpretation of data, statistical analysis and writing of the manuscript with critical review for important intellectual content. DCS, JMSJ, EVC contributed to clinical data acquisition, interpretation, and critical review of clinical data in the manuscript for important intellectual content. ASGA, GAF, TCO contributed to the critically ill patients care in the ICU, blood collection of viable samples for serum antibiotics measurements, and blood collection for biomarkers study. LVKK, TVC and MJS contributed to the review of manuscript and of all articles included in the manuscript, and specially at the last revision done of references included in the manuscript. PR and NJCD contributed to the critical revision of data for important intellectual new contents. PRA and MSS contributed to the discussion of data related to TDM of ATB, and at the critical revision for important intellectual content. All authors read and approved the final manuscript version.
The authors declare that they have no conflicts of interest.

©2024 Santos, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.