eISSN: 2379-6367


Research Article Volume 8 Issue 3
Department of Biochemistry, Federal University Wukari, Nigeria
Correspondence: Ebenezer Ale, Department of Biochemistry, Federal University Wukari, Taraba State, Nigeria, Tel 07038159057
Received: May 27, 2020 | Published: June 18, 2020
Citation: Ale E. Assessment of antioxidant properties of N-Hexane extract of Morinda lucida as a link to its pharmacological actions. Pharm Pharmacol Int J. 2020;8(3):174-178. DOI: 10.15406/ppij.2020.08.00293
Background and objective: Morinda lucidais a flowering plant which belongs to the family, Rubiaceae and reported to possess numerous pharmacological properties. This study was carried out to assess the antioxidant potentials of n-hexane extracts of M. lucida as a link to its medicinal actions.
Methods: The leaves of M. lucida were collected from the local suppliers and the n-Hexane extract of the leaveswas prepared using Soxhlet extractor. The extract was then concentrated in a rotary evaporator and investigated for different antioxidant parameters.
Results: The results of the study revealed that n-hexane M. lucida possess substantial radical scanvenging ability, ferric reducing power, iron chelating ability and antilipid peroxidation property compared to the control (P< 0.05). The documented medicinal properties of the plant can therefore be attributed to the experimentally proved and ascertained antioxidant action.
Keywords: antioxidants, Morinda lucida, n-hexane extract, lipid peroxidation, reactive oxygen species
Reactive oxygen species (ROS) are a class of highly reactive molecules that can cause damage to cells and tissues during various infections and degenerative disorders including cancer.1 ROS occur in tissues participating in potentially deleterious reactions (Paolo et al., 1991) and are capable of damaging DNA, proteins, carbohydrates and lipids in the tissues.2 ROS include superoxide anion, hydrogen peroxide, hydroxyl radical and singlet molecular oxygen. A free radical is any chemical species that can exist independently and possesses one or more unpaired electron.3,4 Lipid peroxidation mediated by free radical is considered to be primarily responsible for cell membrane destruction and cell damage leading to tissue injury and failure of the antioxidant defence mechanism (Comporti, 1989).
An antioxidant is defined as a molecule capable of slowing or preventing the oxidation of other molecules. Antioxidants acts as radical scavenger and inhibit lipid peroxidation and other free radical-mediated processes, thereby protecting the human body from various diseases (Czinner et al., 2001). Natural antioxidants, such as vitamin E (tocopherol), vitamin C, and polyphenols/flavonoids, have been investigated for their possible use to prevent different diseases.5 Recently, polyphenols/flavonoids found in plants are now being investigated and considered by researchers as a new natural antioxidant. Consequently, numerous reports on natural antioxidants, including polyphenols, flavonoids, vitamins, and volatile chemicals have been reported.6
Morinda is a genus of flowering plants in the madder family, Rubiaceae.7 Morinda lucida is a tropical West Africa rainforest tree also called Brimstone tree. In South–Western Nigeria, it is called Oruwo.7,8 It is a medium sized tree at maturity. Different parts of M. lucida have been reported to possess medicinal properties. The leaf extract of the plant was reported to possess trypanocidal,9 antimalarial activities10,11 and aortic vasorelaxant effect.12 The weak decoction of the stem bark has been documented to possess anti-jaundice property.13 Studies also revealed that M. lucida leaf extract possess strong oral hypoglycemic property.7,14 The leaf extract of M. lucida has also been documented to possess anti-spermatogenic activities in rats.15 Its stem bark infusion is used an antimalarial and antidiabetic agent. It showed significant antimalarial activity9,10,11,16-20 and anti-Salmonella typhi activity,18 M. lucida extracts induced contractility effect on isolated uterine smooth muscle of pregnant and non-pregnant mice.11,12 The root of M. lucida is used as chewing sticks for oral hygiene in Nigeria. The aqueous extracts of different parts of this plant were found to be effective against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Pseudomonas aeruginosa.21-23 This present study sought to assess the antioxidant potentials of n-hexane extracts of M. lucida as the ratinale behind its myriads of pharmacological activities.
Sample collection
The leaves of M. lucida were collected from the local suppliers in Akure, Ondo state (Nigeria). The plants collected were identified botanically in Department of Biological Science, Federal University, Wukari. Fresh and tender leaves of selected plants were air-dried for two weeks at 250C. The dried leaves were powdered with mechanical grinder to obtain coarse powder and used for antioxidant assays.
Chemicals
Potassiumpersulphate (K2S2O8), 2,2’-azinobis-3ethylbenzothiazoline-6-sulfonic acid (ABTS), 1, 1-diphenyly-2-picrylhydrazyl (DPPH), iron (II) sulphate, thiobarbituric acid (TBA) were obtained from Sigma (St. Louis, MO). All chemicals used were of analytical grade and were obtained from standard commercial suppliers.
Preparation of n-Hexane extract
The n-Hexane extract of M. lucida was prepared using Soxhlet extractor. 50.0g of pulverized leaves was put into thimble and boiled in n-hexane on a heating mantle at the temperature of 680C. The hexane boiled and washed the extract into the round bottom flask. It was allowed to siphon six (6) times. The n-hexane extract was then concentrated in a rotary evaporator and the n-hexane used for the extraction was recovered. The dried extract was stored in refrigerator for further use.
Antioxidants assays
Determination of ABTS radical scavenging ability
Antioxidant activity of the extract was determined using the 2,2’-azinobis-3- ethylbenzothiazoline-6-sulfonic acid(ABTS) antiradical assay.24 The ABTS•+ (working solution) was prepared by mixing equal volumes of 7mM ABTS and 2.45mM potassium persulphate (K2S2O8) (both prepared using distilled water) in a volumetric flask, which was wrapped in foil and allowed to react for a minimum of 12hrs in the dark. The working solution (900µl) was added to the n-hexane extracts (300µl). The test tubes were allowed to stand for exactly 30min. The absorbance of the standards and samples was read at 700nm in a spectrophotometer.
1,1-diphenyl-2 picrylhydrazyl (DPPH) radical scavenging ability
The free radical scavenging ability of the extracts against DPPH free radical was evaluated as described by Gyamfi et al.25 Briefly, five concentrations (1ml) of the extract were added to 1ml of 0.2Mm methanolic solution containing DPPH radicals, the mixture was left in the dark for 30mins and the absorbance was taken at 517nm. The DPPH free radical scavenging ability was then calculated.
Determination of ferric reducing antioxidant power (FRAP)
The reducing power of the extracts was determined by assessing the ability of the extract to reduce FeCl3 solution in the presence of TPTZ as described by Nair et al., (2007). 100µl aliquot extracts was mixed with FRAP solution containing 100ml of 30mM acetate buffer, 10ml of 10mM TPTZ, 40mM HCl and 10ml of FeCl2. The mixture was incubated at 370C for 30min, and the absorbance was measure at 593nm and ferric reducing power was calculated.
Fe2+ Chelating assay
The Fe2+ chelating ability of the extract was determined using a modified method of Puntel et al.26 168µl 0.1M Tris-HCl (pH 7.4), 218µl of Saline and extract were added to freshly prepared 500µM FeS04 (150µl). The reaction was incubated for 5min followed by addition of 13µl 0.25 1,10-phenanthroline (w/v). The absorbance was subsequently measured at 510nm in a spectrophotometer. The Fe2+ chelating ability was subsequently calculated as percentage (%).
Thiobarbituric acid reactive species (TBARS) assay
Rat was decapitated under mild ether anaesthesia and the liver was quickly removed, placed on ice and homogenized in cold 5mM Tris-HCl pH 7.4. The homogenate was spinned at 4000r/m for 10min in cold centrifuge to yield a low speed supernatant fraction that was used for lipid peroxidation assay. The lipid peroxidation was carried out by measuring TBARS (thiobarbituric acid reactive species) using the modified method of Ohkawa et al.27 An aliquot of 100µl of homogenate was incubated for 1hour at 370C in the presence of extract (final concentrations range of 0-100 mg/ml), in a reaction system containing 50 mM Tris-HCl buffer (pH 7.4), 10µM FeSO4 (prooxidant). The colour reaction was developed by adding 200µl of 8.1% SDS (Sodium Dodecyl Sulphate) to the reaction mixture, this was subsequently followed by the addition of 500µl of acetate buffer, pH 3.4, and 500µl of 0.8% TBA. This mixture was incubated at 1000C for 30 minutes. TBARS produced was measured at 532 nm in UV-visible spectrophotometer.
ABTS radical scavenging ability
The result in figure 1 reveals that the extract reduced ABTS radical by scavenging it in concentration dependent manner. This scavenging ability is significant (P<0.05) when compared with the control, with the highest concentration having the least amount of unscavenged radicals.
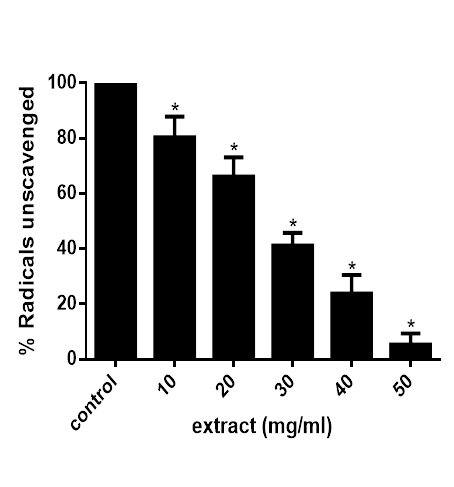
Figure 1 ABTS radical scavenging abilities of extracts of M. lucida. Data are presented as mean±SEM of three independent experiments. *Represent significant difference from control at p<0.05.
1,1-diphenyl-2 picrylhydrazyl (DPPH) radical scavenging ability
Figure 2 shows that at concentration above 10mg/ml, the extract significantly (P<0.05) reduced DPPH· in the reaction medium in concentration dependent manner as observed from the decolourization of the purple colour of DPPH. This indicates that the extract possessed an appreciable DPPH· scavenging ability.
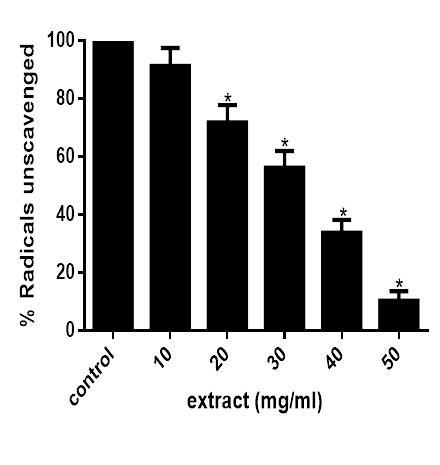
Figure 2 DPPH radical scavenging abilities of M. lucida. Data are presented as mean±SEM of three independent experiments. *Represent significant difference from control at p<0.05.
Ferric reducing antioxidant power (FRAP)
As revealed in figure 3, the extract significantly (P<0.05) reduced Fe3+ to Fe2+ at concentration of 10mg/ml upward as observed from the formation of intense blue colouration. This indicates that the extract possessed a perceptible ferric reducing ability.
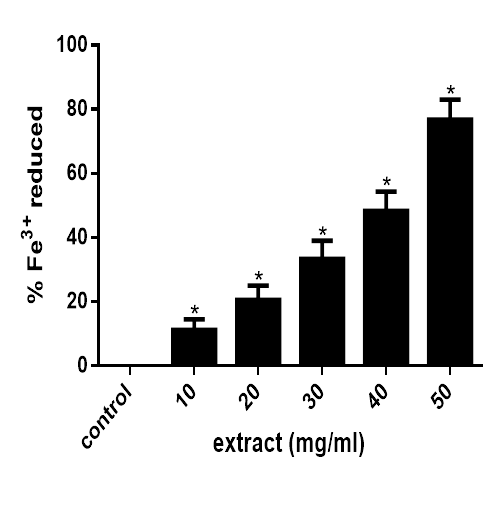
Figure 3 Ferric reducing antioxidant power of M. lucida. Data are presented as mean±SEM of three independent experiments. *Represent significant difference from control at p<0.05.
Iron chelating property
The Fe2+ chelating ability of the extract is presented in figure 4. The result reveals that the extract possess a potent (P<0.05) Fe2+ chelating ability compared to the control.
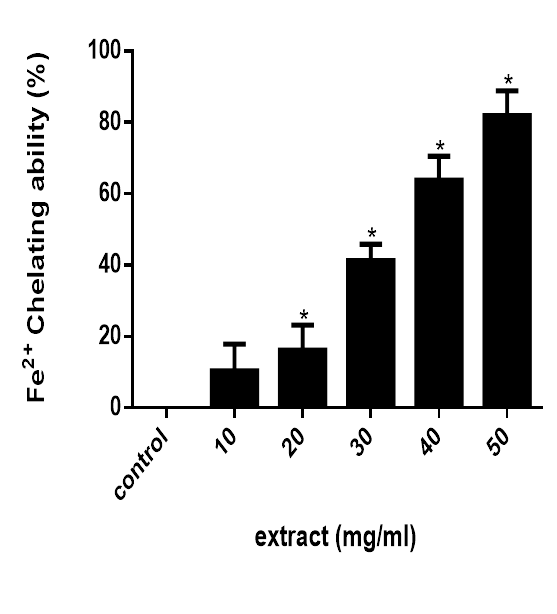
Figure 4 Fe2+ chelating ability of M. lucida. Data are presented as mean±SEM of three independent experiments. *Represent significant difference from control at p<0.05.
Effect of n-hexane extracts TBARS production
As shown in figure 5, the extract inhibited Fe2+-induced lipid peroxidation in rat liver homogenate and the inhibition was significant (P<0.05) when compared to the control. This indicates that the extract potently prevents the formation of TBARS in rat liver homogenate.
Reactive oxygen species (ROS) are a class of highly reactive molecules that can cause damage to cells and tissues during various infections and degenerative disorders including cancer.1 Due to the critical role of scavenging free radicals including ROS by antioxidants, this property in medicinal plants is considered very relevant. M. lucida is a neutraceutical plant used locally for a variety of pharmacological actions such as anticancer, hepatoprotective, cytotoxic, and genotoxic, anti-spermatogenic, hypoglycemic and anti-diabetic activities.28 Herein, different antioxidant parameters were carried out to shed light on the antioxidant efficacy of M. lucida as a link to its reported medicinal values.
The ABTS assay requires that 2, 2’-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) which on treatment with sodium/potassium persulphate29 or MnO230 give a bluish-green radical cation (ABTS+.). The radical cation is reduced in presence of hydrogen donating antioxidants.30,31 The result in figure 1 reveals that the extract reduced ABTS radical by scavenging it in concentration dependent manner with the highest concentration having the least amount of unscavenged radicals.
Moreso, DPPH. (2, 2-diphenyl-1-picrylhydrazyl) is a stable radical owing to stabilization by delocalization on to aromatic rings. DPPH· can trap other radicals easily but does not dimerize. Because a strong absorption band is centered at about 515 nm, the solution of DPPH radical form a deep violet colour and it becomes colorless to pale yellow when reduced upon reaction with hydrogen donor. In similar manner to that of the ABTS assay, the result of the DPPH assay (figure 2) shows that at concentration above 10mg/ml, the extract significantly (P<0.05) reduced DPPH· in the reaction medium in concentration dependent manner. This indicates that the extract possessed an appreciable DPPH· scavenging ability.
Moreover, FRAP assay uses antioxidants as reductant in a redox-linked colorimetric method, employing an easily reduced oxidant system present in stoichiometric excess. At low pH (3.6), reduction of ferric tripyridyl triazine (Fe3+-TPTZ) complex to ferrous form (which has an intense blue colour) can be monitored by measuring the change in absorption.32 As revealed in figure 3, the extract significantly (P<0.05) reduced Fe3+ to Fe2+ at concentration above 10mg/ml indicating that the extract possessed a perceptible ferric reducing ability.
Furthermore, another mechanism of antioxidant action is chelation of transition metals preventing catalysis of hydroperoxide decomposition and Fenton type reactions.33,34 In the presence of chelating agents, complex formation is disrupted leading to colour reduction. Measurement of colour reduction allows the estimation of the chelating activity of the coexisting chelator. Fe2+ possess the ability to move single electron by virtue of which it can allow the formation and propagation of many radical reactions.35,36 Figure 4 reveals that the extracts possess high Fe2+ chelating ability compared to the control.
Moreover, lipid peroxidation, a well-established mechanism of cellular injury in plants and animals, is an indicator of oxidative stress in cells and tissues. Lipid peroxides are unstable and decompose to form a complex series of compounds including reactive carbonyl compounds. Polyunsaturated fatty acid peroxides generate malondialdehyde (MDA) and 4-hydroxyalkenals (HAE) upon decomposition and their measurements are indicator of lipid peroxidation. Lipid peroxidation assay measures the inhibition of production of thiobarbituric acid reactive substances (TBARS) from sodium benzoate under the influence of the free oxygen radicals derived from Fenton’s reaction. The reactive oxygen species degrade benzoate, resulting in the release of TBARS. Antioxidants from the added sample cause suppression of the production of TBARS.37 As shown in figure 5, the extract inhibited lipid peroxidation in rat liver homogenate and the inhibition was significant (P<0.05) when compared to the control. This indicates that the extract impedes the formation of TBARS in rat hepatocytes.
Worth mentioning is the fact that medicinal plants contain varieties of bioactive compounds that account for their therapeutic values. Bioactive compounds are known to have different biological activities including free radical scavenging and antioxidant potential.4,9,38 Studies have revealed that the n-hexane extract of the leaves of M. lucida is rich in phytochemicals such as flavonoids, phenolics, tannins, saponins, steroids and cardiac glycosides.39 Research by40 showed that the presence of flavonoids in the plants may contribute to their antioxidant activity, since flavonoids have been reported to possess strong antioxidant activity. Flavonoids have also been documented to be anti-allergies, antiinflammatory, free radical scavengers, hepatoprotector, anti-microbial, anti-ulcer, anti-viral and affecting platelet aggregation.41,42 Flavonoids are also known to protect the vascular system and strengthen the tiny capillaries that carry oxygen and essential nutrients to all cells. It is on this note, rational to attribute the observed antioxidant properties of M. lucida herein to the reported bioactive compounds present in the plant. Previous reports26,43 also revealed that M. lucida contains alkaloids which confer its effectiveness against pathogenic microorganism. The plant was also reported to be a domicile of several phenols26,41,44 which are modifiers in the pathways of prostaglandin and thereby protect against the clumping of platelets,45,46 they also have the ability to block the uptake of cholesterol and facilitate its excretion from the body. It is therefore evident in this research that the reported therapeutic properties of M. lucida can be attributed to the observed antioxidant actions conferred on it by the resident bioactive compounds.47,48
This study revealed that n-hexane M. lucida possesses strong radical scanvenging ability, ferric reducing power, iron chelating ability as well as substantial antilipid peroxidation property. The documented medicinal properties of the plant can therefore be linked to its antioxidant action. Further research is needed to evaluate the efficacy M. lucida in the treatment and management of oxidative stress-linked diseases such as inflammations, tumors, cancers and diabetes.
None.
Author declares that there is no conflict of interest.

©2020 Ale. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.