eISSN: 2379-6367


Short Communication Volume 7 Issue 6
IFBV-BELHERB, Luxembourg
Correspondence: Pierre Lutgen, IFBV-BELHERB, BP 98 L-6905, Niederanven, Luxembourg
Received: October 27, 2019 | Published: December 6, 2019
Citation: Lutgen P. Artemisia afra and hypertension. Pharm Pharmacol Int J. 2019;7(6):297-300. DOI: 10.15406/ppij.2019.07.00267
Artemisia annua and Artemisia afra have shown a high efficacy in many in vivo and in vitro trials against tropical diseases. The plants also have a hypotensive effect. This paper proposes several hypotheses to explain this property.
Keywords: hypertension, scopoletin, Artemisia afra, nitric oxide, potassium, blood pressure, malaria
Hypertension is a major healthcare problem afflicting nearly 50 million individuals in the United States alone. Hypertension increases the risk of stroke, heart disease, kidney failure and eventually malaria. Artemisia plants have shown a remarkable effect against several tropical diseases: malaria, schistosomiasis, Chagas, leishmaniasis.1‒7
Considering all these data it would not be surprising to find that these diseases and Artemisia plants also have an effect on some metabolic and bodily functions.8 Epidemiological links between malaria parasitaemia and hypertension. A recent paper from South Africa reports a strong antihypertensive effect of Artemisia afra. The extract had its greatest antihypertensive effect at 2 and 4 hours post treatment, while the effect of Leonotis leonuras, another plant used for its antihypertensive properties, and of Furosemide were weak at its best. The authors also found that the Artemisia afra plant extract was non-toxic with LD50 values greater than 5000mg/kg.9,10 Similar hypotensive effects have been noticed in other Artemisia plants, for example, Artemisia herba alba11,12 or Artemisia Persia13 or Artemisia copa in Argentina14 and even Artemisia vulgaris.15
Artemisia maritima was investigated in Tunesia, measuring the effect of the water-boiled extract of Artemisia campestris on human hemodynamic system. To do so, the blood pressure parameters of two groups of adult volunteers were recorded before and each 15 minutes after drinking 20 ml of the boiled extract (20g of dried leaves / 1 L of water). The diastolic pressure and heart rate significantly diminished following the treatment, the systolic pressure did not significantly change.16 Some studies have shown a link between inflammation and hypertension. Human data support previous evidence from animal studies that the cytokine IL-6 plays a role in hypertension. Patients with pulmonary arterial hypertension had significantly elevated IL-1β and IL-6 in the serum.17‒19 A study from the University of Louvain has shown that Artemisia annua infusions reduce IL-6 and IL-8 cytokines. For some unknown reasons the plant from Luxembourg compared with 6 others had the strongest effect. In South Africa Artemisia afra is a well-known herbal medicine used for various inflammatory conditions.20‒23
Hypertensives have higher interleukin-6 as per a study from Boston as shown by this graph
The scopoletin hypothesis
Already in 1983, the plausible mechanisms of the hypotensive effect of scopoletin, a coumarin have been investigated in vivo and in vitro. The results obtained show that scopoletin probably produces hypotension in laboratory animals through (a) its smooth muscle relaxant activity, which means it presumably dilates blood vessels; and (b) by acting as a non-specific spasmolytic agent.24 The same hypothesis was developed in a paper from Ghana. Their results suggest that aqueous extracts of Artemisia afra are potentially useful for the management of hypertensive condition.25 The blood pressure lowering effect of scopoletin is well described in a paper from Indonesia (Figure 1).26

Figure 1 Comparative effect of the treatment to MAP (mean arterial pressure) on different hypersensitive models during 120 minutes of observation (PN= group treated with prednisone + Nacl; PNL= group treated with prednisone + Nacl + L-NAME; data with negative (-) values indicate the decrease of the parameter; *p<0.05 and ns = not significant comparative to control; n=18).
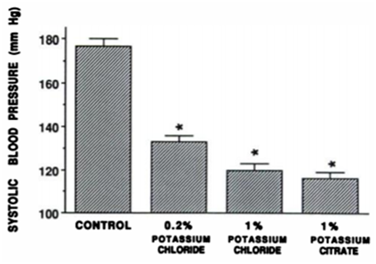
Figure 2 Changes in systolic blood pressure in DOCA-salt hypertensive rats receiving 1% sodium chloride to drink.
Scopoletin was one of the first major constituents detected in Artemisia afra some 100 years ago.27 Artemisia afra is rich in scopoletin 0.10 mg/gDW vs 0.05-0.06 in Artemisia annua.28 The Noni fruit which is rich in scopoletin is reputed for its hypotensive activities.29
The arginine hypothesis
Reduced nitric oxide (NO) bioactivity represents prominent pathophysiological abnormalities associated with hypertensive cardiovascular disease. L-arginine serves as the principal substrate for vascular NO production. Several studies demonstrate a beneficial effect of chronic or acute L-arginine supplementation. One of the possible mechanisms is vascular dilatation.30,31 A meta-analysis included 11 randomized, double-blind, placebo-controlled trials involving 387 participants with oral L-arginine intervention ranging from 4 to 24 g/d. Compared with placebo, L-arginine intervention significantly lowered systolic BP by 5.39 mm Hg diastolic BP by 2.66 mm Hg. There is indeed an abundant literature on this topic.32‒36
Suppression of plasma nitric oxide (NO) concentration may be involved in the pathogenesis of cerebral malaria, according to the authors of a combined USA/Tanzanian study. Tanzanian children with cerebral malaria had significantly decreased plasma and urine nitrogen oxide concentrations compared with those with symptom-free parasitaemia and healthy controls. In-vitro work has also previously supported an anti-plasmodial role for endogenous NO-related compounds.37 A recent study from Ukraine has analyzed the amino acid content in some 8 plants of the Artemisia subgenus found that they are all 5 to 10 times richer in arginine than other herbs or vegetables, with A. annua top-ranking (2g/100g).38 This is in line with the results of Brisibe and Ferreira who also find 2g/100g for Artemisia annua. Onions contain 0.10 g/100g, garlic 0.63 g/100 and milk 0.2g/100g.39
The potassium hypothesis
Epidemiologic, experimental, and clinical studies have shown that potassium is an important regulator of blood pressure.40‒43 Potassium intake has been shown to have a beneficial effect on the cardiovascular system. Increased potassium swells endothelial cells and modifies endothelial cell stiffness.44 Artemisia plants are very rich in potassium and contain virtually no sodium. The work of Brisibe and Ferreira has found this for Artemisia annua (E A Brisibe, op.cit.). Similar high concentrations of potassium have been found in Artemisia herba alba, and a barely detectable presence of sodium.45
The role of artemisinin: beneficial or detrimental?
It is strange that Artemisia annua or artemisinin are never mentioned to have a hypotensive effect. An increasing body of evidence suggests that oxidative stress, which results in an excessive generation of reactive oxygen species (ROS), has a key role in the pathogenesis of hypertension. These reactive oxygen species include superoxide, hydroxyl radical, hydrogen peroxide, peroxynitrite, hypochlorous acid and artemisinin. These strong oxidants also kill the plasmodium parasite.46 The pro-oxidant activity of artemisinin is simply too potent and rapid for wild-type parasites to combat and survive.47 Clinical studies demonstrated increased ROS production in patients with essential hypertension, renovascular hypertension, malignant hypertension.48
Malaria and hypertension
Some studies show that there are indications of a potentially dangerous link between high rates of malaria and high blood pressure, and they are urging more research in hopes of better addressing harmful effects of hypertension in malaria–stricken areas. Some studies find that malaria raises blood pressure.49,50 Some other studies find that malaria lowers blood pressure.50,51 But the effect of high blood pressure on malaria immunity or sensitivity has barely been studied.
This obviously is a new research field of a high complexity. The effect of Artemisia plants on hypertension is well described in the scientific literature. But we are lacking an understanding of the mechanism for the hypotensive effects as we still do for the antimalarial effects. It is a polytherapy and each attempt to isolate one molecule and to claim that the efficacy of the plant is due to this monotherapy has failed. This is for example in malaria infections the case for quinine and its derivative chloroquine or for artemisinin and its derivative artesunate. Even for artemisinin alone the mechanism of action has not been fully elucidated and five hypotheses are still in debate.
|
Artemisia constituents which have been reviewed in this paper |
Artemisia constituents which deserve further investigation |
|
Scopoletin |
Essential oils |
|
Potassium |
Polycyclic triterpenes |
|
Arginine |
Anthocyanidins |
|
Artemisinin |
Saponins |
|
|
Arachidonic acid |
The interplay between molecules or minerals in a plant can be synergistic or antagonistic. What is found in vitro often does not work in vivo. The aim of our research team is to better understand these interactions, and explanations found in a field like hypertension might also apply to malaria or other tropical diseases. We are presently working with 3 universities in Belgium and 8 universities in Africa on the topic of plant polytherapy. Several laboratories and medical teams have studied the eventual toxicity of Artemisia annua or Artemisia afra and their interaction with other drugs. A review of all the scientific papers on these topics has been published. Nobody was able to detect any acute or chronic toxicity for the consumption of these plants, which have been utilized since millenaries without any side effect.
None.
Author declares that there is no conflict of interest.

©2019 Lutgen. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.