eISSN: 2379-6367


Research Article Volume 8 Issue 4
1State University of Ceara, Science and Technology Center (CCT), Laboratory of Natural Products Chemistry - Brazil
2State University of Ceara, Department of Chemistry, Laboratory of Natural Products Bioprospecting and Biotechnology, Brazil
3University of Fortaleza, Experimental Biology Nucleus (NUBEX), Brazil
4State University of Vale of Acarau, Chemistry Course, Laboratory of Natural Products Chemistry, Synthesis and Biocatalysis of Organic Compounds-LBPNSB, Brazil
Correspondence: Helcio Silva dos Santos Science and Technology Center-Chemistry Course, State 21 University of Vale do Acarau, Sobral, Ceara, Brazil, Tel +55-85-3366-8300
Received: July 31, 2020 | Published: August 31, 2020
Citation: Silva AWD, Ferreira MKA, Reboucas EL, et al. Anxiolytic-like effect of Azadirachta indica A. Juss. (Neem, Meliaceae) bark on adult zebrafish (Danio rerio): participation of the Serotoninergic and GABAergic systems. Pharm Pharmacol Int J. 2020;8(4):256-263. DOI: 10.15406/ppij.2020.08.00303
Keywords: neem barks, acute and chronic anxiety, adult zebrafish, GABAA, 5-HT
Anxiety disorders are among the top ten diseases responsible for disability worldwide, and Brazil, in 2015, was considered by the World Health Organization (WHO) the country with the highest rate of anxiety disorders in the world, with 9.3% of the population.1
Abstinence-induced anxiety is a common problem in drug abuse.2 This problem is associated when consumption is discontinued or abruptly reduced, with the occurrence of symptoms such as trembling, anxiety, insomnia, agitation, hypervigilance, irritability, piloerection and, sometimes, seizures.3 Benzodiazepines (GABA receptor agonists) and selective serotonin reuptake inhibitors (SSRIs) are the drugs of choice for the treatment of anxiety.2 SSRIs are also commonly used to treat depressive disorders.4 However, the chronic use of benzodiazepines causes tolerance, and abrupt treatment discontinuation may lead to withdrawal syndrome.5 On the other hand, the chronic use of SSRIs can result in considerable side effects,6 therefore the search for new compounds with anxiolytic and antidepressant properties that cause fewer adverse effects continues.7
Animal models are used for the assessment of new anxiolytic drugs. These pre-clinical models and screening tests support the studies, since clinical trials are expensive,8 especially regarding the central nervous system therapy.9 Currently, the zebrafish (Danio rerio) has been used in behavioral neuroscience, including research involving the brain and psychopharmacology.9 This vertebrate animal is considered a significant model in preclinical studies, because its genotype has 70% homology with mammalian neurotransmitter receptors.10
Because of the side effects associated with allopathic drugs, there is a growing interest in the development of alternative therapies to treat psychiatric disorders.11 Previous studies have demonstrated the anxiolytic action of several phytochemical groups, such as polyphenols and flavonoids found in plants.12 In this context, Azadirachta indica A. Juss. (Neem), which belongs to the Meliaceae family, native to India, and also found in Brazil, is rich in flavonoids.13 Both the bark and leaves of A. indica contain biologically active molecules, which have hypolipidemic, hypoglycemic, immunostimulatory, hepatoprotective, anti-inflammatory and antifertility properties.14 A. indica limonoids and phytosterols play an important role, having anti-inflammatory, immunomodulatory and antioxidant properties.15 Previous study carried out with A. indica leaf extracts have demonstrated anxiolytic action in rats.16
Based on the abovementioned facts, this study aimed at evaluating the anxiolytic-like effect of A. indica A. Juss bark and possible mechanisms of action in adult zebrafish (Danio rerio).
Drugs and reagents
The following reagents and drugs were used in the study: commercial ethanol (96%), ethyl alcohol PA (99.5%; Biotec®), KOH (99% m/m; Vetec®), HCl (36.5% v/v; Microquímica®); Ferric chloride, gallic acid, KBr, 2,2-diphenyl-1-picrylhydrazyl (DPPH), Tween 80, flumazenil (Sandoz), granisetron hydrochloride (Kytril), dichloromethane, ethyl acetate, Folin-Ciocalteu, pizotifen maleate (Sandomigran®), fluoxetine (Teuto), Cyproheptadine (Cobavital®) and Diazepam (Teuto).
Botanical material
The A. indica bark was collected in the city of Tauá (040º18’05,4” W; 06º01’03,6” S) state of Ceará, Brazil, after obtaining authorization from SISBIO, according to the registration for the collection of botanicals, fungal and microbiological material, nº 29145-4. The botanical identification was carried out in the Prisco Bezerra Herbarium of the Federal University of Ceará, where an exsiccate was deposited under number 56044.
Extract preparation
The crude extract was obtained from the bark (350g) of Azadirachta indica (Neem, Meliaceae), using the methodologies described by Matos.17 Commercial ethanol (96 °GL) was used as the organic solvent, and after 96 hours of cold extraction, simple filtrations were performed, and the extracts were kept in a water bath (50±2°C) for complete solvent evaporation. The yield of the obtained Neem bark ethanolic extract (EtCNeem) was 89.4 g (25.54%).
Saponification reaction
The saponification reaction was performed following the methodology proposed by Scroder.18 EtCNeem (5 g) was dissolved in an alcoholic solution (ethyl alcohol P.A. 99.5% Biotec®) of Vetec KOH (99%; m/m). A thermostatic water bath was used at 60ºC for 60 minutes for the saponification reaction of the sample fatty acids. After the completion of the reaction, the material was transferred to an ice bath and after it cooled, 36.5% HCl (pH 1.0) was added for complete release of the fatty acids from their respective salts. The unsaponifiable organic compounds were extracted using dichloromethane and ethyl acetate, then separated from the residual fraction (saponifiable compounds or fatty acids) through the settling funnel. The yields of the unsaponifiable fractions dichloromethane (F-Dic) and ethyl acetate (F-EtOAc) obtained were 2.56 g (10.24%) and 0.62 g (2.48%), respectively. A yield of 21.82 g (87.28 %) was obtained from the saponifiable fraction (F-Sap).
Antioxidant activity-DPPH Free Radical Scan
A methanol solution of DPPH was added to the methanolic solutions of the samples (10 to 10.000μg/mL). The test was performed in triplicate. After the 60-minute interval, absorbance was measured in a UV-Vis spectrophotometer at 515 nm. Pearson’s correlation (r) was used to evaluate the association between phenol and flavonoid contents with antioxidant activity. The antioxidant capacity was compared with a standard quercetin curve and EC50 was determined.19
Preliminary phytochemical screening
The fraction with the best antioxidant activity (F-EtOAc, see Results section) was submitted to a preliminary phytochemical screening to detect the major classes of secondary metabolites through chemical reactions that result in color changes and/or result in the formation of a precipitate specific to each class of substances, using the methodologies described by Matos.17
Fourier Transform Infrared Spectroscopy (FT-IR)
Fourier Transform Infrared Spectroscopy (FT-IR) was performed to identify the possible functional clusters present in F-EtOAc. The FT-IR data were obtained using an IRTracer-100 Spectrometer (Shimadzu, Japan), in which 2 mg of the sample was dispersed in 200mg of KBr and pressed at 80 kN to form the KBr pellets, and then analyzed in the range of 400-4000 cm-1 with a resolution of 4 cm-1 and 64 scans per minute.
Measurement of the total phenolic compounds
The measurement of the total phenolic compounds of F-EtOAc was performed using the Folin-Ciocalteu method,20 where a calibration curve was prepared using diluted solutions of gallic acid (0-500μg/mL). The total phenol (TP) content was determined by interpolating the absorbance of the samples against a calibration curve constructed with gallic acid standards and expressed as mg of EGA (equivalent of gallic acid) per 100 g of extract. The equation for the gallic acid calibration curve was C=0.0009A, where C represents the concentration of gallic acid, A represents the absorbance at 750 nm and the correlation coefficient R=0.9916. The TP means, and the respective standard deviations were submitted to analysis of variance (ANOVA) using the software GraphPadPrim. Significant differences (p<0.05) between the means were determined by Tukey multiple comparison test.
Estimation of total flavonoids content
The total flavonoids content of the extract was analyzed utilizing aluminum chloride (AlCl3) method.21 The calibration curve was prepared with quercetin [500–31.25μg/mL in ethanol at 80 % (v/v)]. Samples of 0.5 mL of each extract (1 mg/mL) or standard solutions were united with 1.5 mL of ethanol at 95 % (v/v), 0.1 mL of AlCl3 at 10 % (w/v), 0.1 mL of sodium acetate at 1 mol/L and 2.8 mL of distilled water. In the blank test, the volume of aluminium chloride at 10% was substituted by distilled water. The incubation occurred at room temperature for 30 min and the absorbance reading was measured at 415 nm in a spectrophotometer. The measures were performed in triplicate and the average value was expressed in μg of quercetin equivalents (QuerE) per milligrams of extract (μg/mg).
Toxicity to Artemia salina
The solutions of EtCNeem and fractions (100, 500 and 1000μg/mL) were tested in triplicate. The surviving nauplii were counted after 24 hours and the percentage of dead nauplius was determined by calculating the lethal concentration that will kill 50% (LC50) using the Probit analysis with 95% confidence. The toxicity potential of the substances was classified as: a) Non-toxic (LC50> 1000μg/mL); b) Toxic (LC50 ≤ 1000μg/mL).
Adult Zebrafish (Danio rerio) (aZF)
Wild adult zebrafish (60 to 90 days old; 0.4±0.1 g, 3.5±0.5 cm), of both genders, were obtained from a commercial supplier (Fortaleza, CE). Groups of animals (n = 30) were acclimatized for 24 h in a 10 L glass tank (30 × 15 x 20 cm) containing dechlorinated tap water (ProtecPlus®) and a submersible filter and air pump at 25°C and pH 7.0, under a circadian cycle of 14:10 h (light/dark). Animals received ad libitum feed 24 h prior to the experiments. After the experiments, the animals were euthanized by immersion in ice water (2-4°C) in up to 2 minutes until loss of opercular movements occurred. All experimental procedures were approved by the Animal Research Ethics Committee of the State University of Ceará (CEUA-UECE # 7210149/2016).
Acute toxicity to adult zebrafish
The study was carried out based on the methodology proposed by Ekambaram et al.,22 with adaptations. As an adaptation of the method, aZF (n=6/group) were treated intraperitoneally (i.p.) with 20μL of the best antioxidant fraction (1.0; 2.5 and 5.0 mg/mL) of non-toxic samples against A. salina (see Results section). As negative control, 3% Dimethyl sulfoxide (DMSO) (20μl, i.p.) was used. After 24, 48, 72 and 96 hours, the values obtained regarding the number of dead aZF were submitted to statistical analysis, estimating the lethal dose to kill 50% (LD50) of aZF utilizing the Trimmed Spearman-Karber method with 95% confidence intervals.
Evaluation of locomotor activity
The Open Field Test proposed by Magalhães et al.,23 was used to evaluate whether or not there was alteration of the motor coordination in the animals. aZF (n=6/group) were treated intraperitoneally (i.p.) with F-EtOAc (1.0 or 2.5 or 5.0 mg/mL, 20μL; i.p.) or vehicle (DMSO 3%; i.p.) or diazepam (Dzp1.0 mg/mL, 20μL; i.p.). After 30 min of the treatments, the animals were added to Petri dishes containing the same water of the aquarium, marked with four quadrants and the locomotor activity was analyzed by counting the number of line crossings, during 5 minutes. Animals that did not receive treatments (Naïve) were considered as baseline (100% of locomotor activity).
Test light & dark
The anxiolytic-like effect of F-EtOAc was investigated through the Light & Dark Test, based on the methodology proposed by Gebauer et al.24 The animals (n=6/group) were treated intraperitoneally with F-EtOAc (1.0 or 2.5 or 5.0 mg/mL, 20μL; i.p.) or vehicle (Control, 3% DMSO, 20μL) or diazepam (Dzp; 1.0 mg/mL, 20μL; i.p.). An untreated group (Naïve) was included. After 30 min of the treatments, the animals were added to the light zone of the glass aquarium (30 x 15 x 20 cm), which was divided into light and dark zones, with drug-free water. The anxiolytic-like effect was characterized by the presence of the animals in the light zone, during 5 min of analysis.
Involvement of the serotonergic system
Groups of animals (n=6) received cyproheptadine (5-HT2A antagonist) or pizotifen (5-HT1 antagonist and 5-HT2A/2C) orally (p.o.), either at a dose of 0.8 mg/mL or granisetron (5-HT3A/3B antagonist, 0.5 mg/mL, p.o.)30 minutes before F-EtOAc (1.0 mg/mL, i.p.) or fluoxetine (Flx; 1.25 × 10−3mg/mL, i.p.). Subsequently, the Light & Dark Test was performed as cited previously.
Involvement of the GABAergic system
To evaluate the involvement of the GABAergic system, other groups of animals (n=6/each) received Flumazenil (0.1 mg/mL) 15 minutes before F-EtOAc (1.0 mg/mL) or Diazepam (1.0 mg/mL) and the Light & Dark Test was performed as previously described.
Alcohol withdrawal-induced anxiety
Alcohol withdrawal-induced anxiety was investigated in adult zebrafish, based on the method described by Ferreira et al.25 Yellow sugar cane brandy (YSCB) was used as the source of ethanol (38% EtOH). Then the animals (n=6/group) were divided into seven groups:
After 1 h of the oral treatments and 30 min of the intraperitoneal treatments, the animals were individually added to the light zone of the aquarium and the anxiolytic-like effect was characterized by the permanence of the animals in the light zone during 5 min of analysis. Reversion of anxiety caused by the abstinence crisis was evaluated only on the 11th day, where aZF’s (n = 6/group) received F-EtOAc (1.0 or2.5 or 5.0 mg/mL; i.p.) or Diazepam (1.0 mg/mL; i.p.).
Statistical analysis
Results were expressed as mean±standard deviation of the mean for in vitro tests (n=3) as well as mean±standard error of the mean for in vivo tests (n=6/group). After confirming the distribution of normality and homogeneity of data, differences between the groups were submitted to analysis of variance (one-way ANOVA), followed by Tukey test, using the software GraphPad Prism 7.0. The level of statistical significance was considered at 5% (p<0.05).
Toxicity
EtCNeem was shown to be toxic to A. salina nauplii (LC50=288.46μg/mL). Through the extract saponification, it was observed that none of the obtained fractions caused the mortality of 50% of the A. salina nauplii (LC50 > 1.000μg/mL), in addition to not being toxic in adult zebrafih within 96 h of analysis (LD50> 5.0 mg/mL).
Antioxidant activity
F-EtOAc of EtCNeem showed higher antioxidant potential against DPPH (EC50 = 21.6 ± 0.07μg/mL). Pearson’s correlation coefficient (r) indicated that such antioxidant activity was correlated with the phenol (r = 0.4135) and flavonoid (r = 0.9924) contents in 41 % and 99 %, respectively. That is why this fraction was chosen for testing of anxiolytics.
Preliminary phytochemical screening and FT-IR analysis
The preliminary phytochemical analysis of the F-EtOAc disclosed the presence of phenolic, flavonoid, steroid and triterpenoid compounds. Through FT-IR, the presence of functional groups of these classes of chemical constituents was detected, as shown in Figure 1, Table 1.
Functional group |
Vibration (Stretch/Bend) |
Range (cm-1) |
F-EtOAc_EtCNeem |
Alkanes |
C-H stretch |
3000-2800 |
✔ |
C-H bend |
1500-1440 |
✔ | |
Alkenes |
C-H stretch |
3200-3000 |
- |
Aromatic rings |
C=C stretch |
1600-1400 |
✔ |
Hydroxyl |
O-H stretch |
3600-3200 |
✔ |
Carbonyl |
C=O stretch |
1610-1550 |
✔ |
Amino acid |
C=O stretch |
1660-1600 |
✔ |
Aldehydes |
C=O stretch |
1750-1625 |
✔ |
Amines |
C-H stretch |
2850-2700 |
- |
Nitriles |
C≡N stretch |
2500-2000 |
✔ |
Amides |
N-H stretch |
3500-3100 |
- |
C=O stretch |
1670-1600 |
✔ | |
Ethers |
C-O-C stretch |
1260-1040 |
✔ |
Table 1 List of functional groups of chemical constituents present in Et-EtOAc the EtCNeem
Measurement of total phenols and flavonoids
F-EtOAc is rich in phenolic compounds (454.32±0.005 mg of EGA/g of extract), as well as in flavonoids (41.12±0.045 mg EQuer/g of extract).
Locomotor activity evaluation (Open Field Test)
All doses of F-EtOAc (**p<0.05) and Diazepam (****p<0.0001) decreased the locomotor activity of adult zebrafish in comparison to the naive and vehicle groups (F5,30 = 10.01; Figure 2).
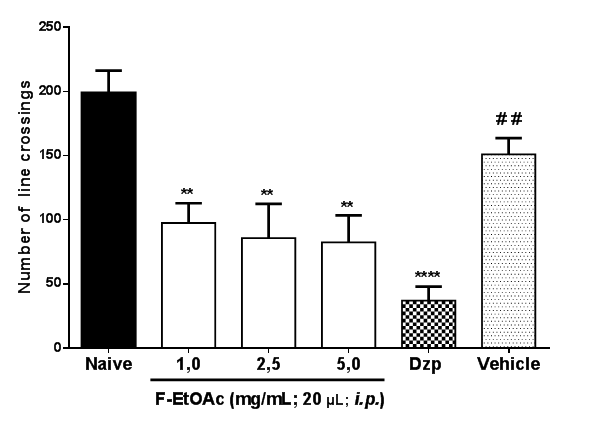
Figure 2 Effect of Et-EtOAc the EtCNeem under the locomotor behavior of zebrafish (Danio rerio) adult in the Open Field Test (0-5 min). F-EtOAc – Fraction ethyl acetate; Dzp – Diazepam (1.0 mg/mL, i.p.); Vehicle (DMSO 3%). Values represent the mean ± standard error of the mean for 6 animals/group; ANOVA followed by Tukey ****p<0.0001, **p<0.01 vs Naive; # #p<0.01 vs Dzp.
Preliminary anxiolytic activity (Light & Dark Test)
The F-EtOAc (1.0 or 2.5 or 5.0 mg/mL; i.p.) and Diazepam (1.0 mg/mL; i.p.) increased (****p< 0.0001 vs naive or vehicle) the permanence in the light zone of the Light & Dark Test (F5, 30 = 60.4; Figure 3).
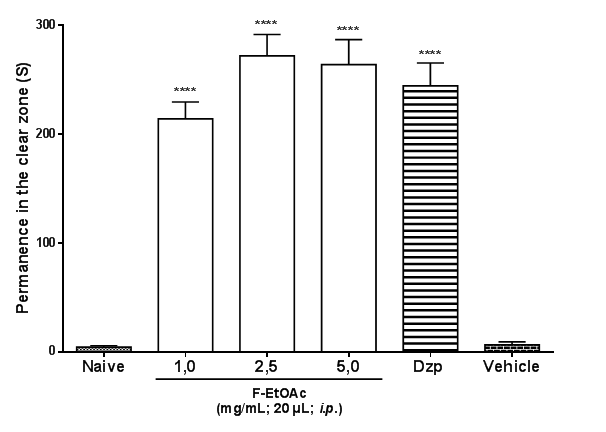
Figure 3 Effect of Et-EtOAc the EtCNeemon adult zebrafish in the Light & Dark Test (0-5 min). The numbers above each column indicate percentage of permanence in the light zone. Naïve–group without treatment; Vehicle–Control (DMSO 3%; 20 μl;i.p.); Dzp–Diazepam (1.0 mg/mL; i.p.). Values represent the mean ± standard error of the mean for 6 animals/group; ANOVA followed by Tukey. ****p<0.0001 vs naive and vehicle.
Involvement of the Serotonergic System (5-HT)
Involvement of the 5-HT2A System
Cyproheptadine not reduced the anxiolytic-like effect of F-EtOAc (1.0 mg/mL, i.p.). However, cyproheptadine (# # # #p<0.0001) reduced the anxiolytic-like effect of fluoxetine (Flx; 1.25×10−3 mg/mL; 20μL; i.p.) (F6,35 = 135.6; Figure 4A).

Figure 4 Anxiolytic-like effect of F-EtOAc after pretreatment with cyproheptadine (A), pizotifen (B), and granisetron (C) in adult zebrafish in the light/dark test (0–5 min). Naive, untreated fishes; Cypro, cyproheptadine (0.8 mg/mL; 20μL; p.o.); Piz, pizotifen (0.8 mg/mL;20μL; p.o.); Gstn, granisetron (0.5 mg/mL; 20μL; v.o.). F-EtOAc (1.0 mg/mL; 20μL; i.p.); Flx, Fluoxetine (1.25 × 10−3 mg/mL; 20μL; i.p.); Vehicle: DMSO 3% (20μL; i.p.). Values are presented as group mean±} S.E.M. Differences between the groups were determined by ANOVA followed by Tukey’s post hoc test. Significant differences: ****p< 0.0001 vs. naive or vehicle; # # # #p< 0.001 vs. Flx or F-EtOAc.
Involvementof the 5-HT1 and 5-HT2A/2C systems
The Pizotifen reduced (# # # #p<0.0001) the anxiolytic-like effect of F-EtOAc (1.0 mg/mL; i.p.) and fluoxetine (Flx; 1.25×10−3 mg/mL; 20μL; i.p.) (F6,35 =132.7; Figure 4B).
Involvement of the 5-HT3A/3B system
The Granisetron reduced (# # # #p<0.0001) the anxiolytic-like effect of F-EtOAc (1.0 mg/mL; i.p.) and fluoxetine (1.25×10−3 mg/mL; 20μL; i.p.) (F6,35 = 137.7; Figure 4C).
Involvement of the GABAergic system
Flumazenil reduced (# # # #p<0.0001) the anxiolytic-like effect of F-EtOAc (1.0 mg/mL; i.p.) and of Diazepam (1.0 mg/mL; i.p.) (F6,35 = 68.49; Figure 5).
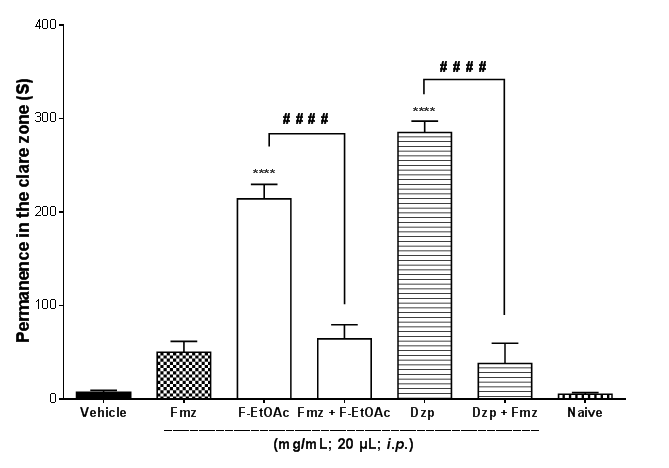
Figure 5 Effect of flumazenil under the anxiolytic-like action of F-EtOAc of EtCNeem in adult zebrafish (Danio rerio) on the Light & Dark Test (0-5min). Naïve–untreated animals; F-EtOAc (1,0 mg/mL, i.p.); Dzp–Diazepam (1,0 mg/mL; i.p.). Fmz–Flumazenil (0,1 mg/mL; i.p.). Vehicle–(3% DMSO 20μl, i.p.). Values represent the mean ± standard error of the mean for 6 animals/group. ANOVA followed by Tukey (****p<0.0001 vs. naive or vehicle; # # # #p<0.0001 vs. Flzor F-EtOAc).
Alcohol withdrawal-induced anxiety
YSCB (38%; 20μL; p.o.) caused an anxiolytic-like effect from the 4th to the 5th day (****p<0.0001 vs. naïve or vehicle; F6,385=58.63) of treatment. This effect was also observed during three days of abstinence: on the 6th and 7th day (****p<0.0001); on the 8th day (**p<0.01) (Figure 6). F-EtOAc (5.0 mg/mL, i.p.) significantly prevented (****p<0.0001) alcohol-withdrawal induced anxiety in adult zebrafish on the 11th day, significantly similar to the effect of Diazepam (Dzp; 1.0 mg/mL; i.p.; ****p<0.0001; F6,35 = 16.86), Figure 7.
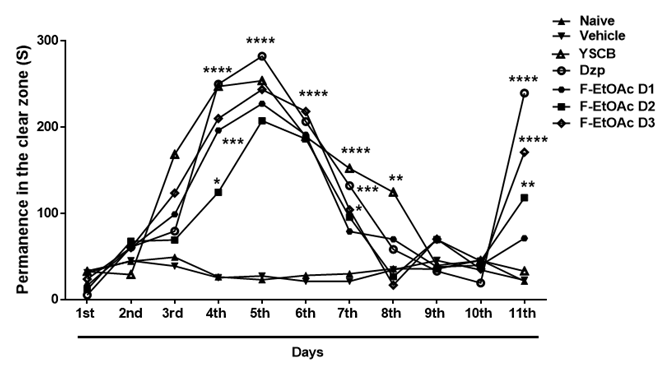
Figure 6 Effect of F-EtOAc on anxiety in adult zebrafish, induced by alcohol withdrawal, in the light and dark test (0-5min). Group I - Naive - untreated animals (Control). Group II - Vehicle (DMSO 3%; 20μl, i.p.; 1st-11th day). Group III - YSCB - yellow sugar cane brandy (EtOH 38%, 20μL; p.o.; 1st-5th day). Group IV - F-EtOAc D1 (1,0 mg/mL; i.p.; 11th day). Group V - F-EtOAc D2 (2,5 mg/mL; i.p.; 11th day). Group VI - EtOAc D3 (5,0 mg/mL; i.p., 11th day). Group VII - Dzp-Diazepam (1.0 mg/mL, i.p.; 11th day).
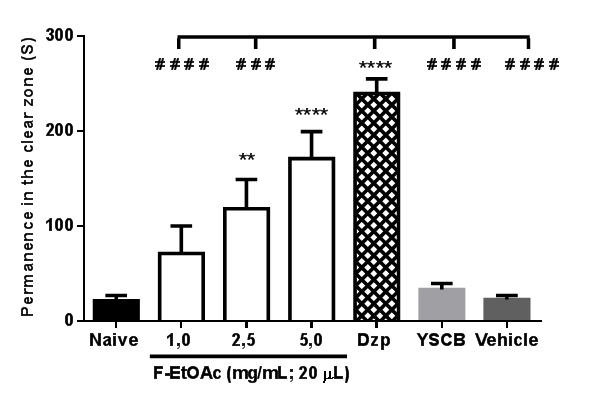
Figure 7 Prevention of anxiety induced by alcohol withdrawal (11th day) by F-EtOAc in the Light & Dark test (0–5 min). The values represent the mean ± standard error of the mean (E.P.M.) for 6 animals/group. Naive - untreated animals. YSCB - yellow cane spirit (38% EtOH); Dzp - Diazepam (1.0 mg/mL; i.p.). Vehicle - 3% DMSO (20μL; i.p.). ANOVA followed by Tukey (**** p<0.0001, **p<0.01 vs. Naïve or YSCB or Vehicle; # # # #p<0.0001 vs. Dzp).
Although the anxiolytic activity of A. indica leaf extracts has already been investigated in rodents,16 the present study is the first to report the ability of A. indica bark ethanolic extract to reverse acute and chronic anxiety in zebrafish adult. The absence of toxicity after the saponification of the Neem bark ethanolic extract indicates its use in pharmacological anxiety tests, since the fractions obtained after the saponification were not toxic for A. salina and adult zebrafish. This absence of Neem toxicity was also demonstrated in the study by Kanagasanthosh et al.26
The phenolic and flavonoid compounds present in ethanolic extracts of medicinal plants constitute classes of secondary metabolites responsible for the antioxidant action against DPPH radicals.27 Our results showed F-EtOAc antioxidant action against the DPPH radical, being the most promising in relation to the presence of phenolic compounds (41.35%) and flavonoids (99.24%).
The antioxidant action of phenolic compounds from medicinal plants has been correlated with antidepressant and anxiolytic potentials.28 In these pathological states, there are higher levels of free radical production in the brain, which results in depletion of the tripeptide glutathione (GSH), a redox regulator, which participates in the maintenance of oxidative homeostasis and reactive oxygen species (ROS) detoxification in brain cells.28 The study by Jaiswal et al.,16 indicated that low doses of A. indica leaf extract produced anxiolytic effects in rodents. In our study, it was possible to detect the anxiolytic-like effect on adult zebrafish of the F-EtOAc (1.0 or 2.5 or 5.0mg/mL) obtained from the Neem bark (Figure 3).
The analysis of the locomotor activity can be used as a hyperactivity evaluation parameter, indicating anxiety. The benzodiazepines used in the treatment of anxiety decrease the locomotor activity of adult zebrafish.2,25,29 The F-EtOAc of EtCNeem had anxiolytic effect on the adult zebrafish, as it reduced the animals’ locomotor activity (Figure 2).30 In order to confirm anxiolytic behavior of F-EtOAc of EtCNeem, the Light & Dark test in an aquarium was performed, one of the methods most frequently used in anxiolytic tests with adult zebrafish (Danio rerio). All doses of EtOAc increased the animals' permanence in the clear area of the aquarium, indicating anxiolytic effect (Figure3).
Serotonin (5-hydroxytryptamine; 5-HT) is a monoamine neurotransmitter, which modulates mood, emotion and defensive, social and anxiety behaviors.31 The genes encoding serotonin receptors in zebrafish show high homology with the corresponding human genes.32 The anxiolytic potential of F-EtOAc was assessed after pretreatment with cyproheptadine antagonists (a 5-HTR2A antagonist), pizotifen (a 5-HTR1 and 5-HTR2A/2C antagonist) and granisetron (an antagonist of 5-HTR3). Fluoxetine was used as a positive control. Figure 4A shows that cyproheptadine did not reverse the anxiolytic effect of F-EtOAc, as the treated animals continued the clear region of the aquarium (****p<0.001 vs. naive/vehicle). Nevertheless, the anxiolytic activity of F-EtOAc was successfully inhibited by pretreatment with pizotifen (Figure 4B) and granisetron (Figure 4C), which reduced the animals' permanence in the clear zone to basal levels (p<0.0001 vs. F-EtOAc) respectively. These results suggest that the mechanism of action of F-EtOAc involves 5-HTR1/5-HTR2C and 5-HTR3, but not 5-HTR2A. Additionally, all antagonists have abolished the effects of fluoxetine (Figure 4 A, B and C).
Flumazenil is a GABAA receptor antagonist with anxiogenic properties,33 responsible for antagonizing the effects of benzodiazepines, such as diazepam, including anxiolytic, sedative and hypnotic effects. In the literature, many researchers have used flumazenil as a tool to identify possible endogenous and exogenous ligands in benzodiazepine receptors in rodents,34 as well as in adult zebrafish.2 In this study, the reversion of anxiolysis through pretreatment with flumazenil suggests that the anxiolytic-like effect of F-EtOAC of EtCNeem depends on the GABARA (Figure 5).
Studies have shown that acute exposure to alcohol acts as a potent anxiolytic, because when it enters the bloodstream, it penetrates the brain and stimulates neurons to release extra serotonin and dopamine, neurotransmitters that regulate pleasure, mood, and anxiety.35 Moreover, alcohol can affect neurotransmitters, such as by inhibiting the glutamatergic system and stimulating the GABAergic,36 initially resulting in a calming effect.37
Sena et al.,38 used distilled cachaça as a source of ethanol for the induction of anxiety in rodents. Benneh et al.,2 used ethanol withdrawal (0.5%; v/v) to induce chronic anxiety in zebrafish by ethanol withdrawal, and then used the Maerua angolensis trunk bark extract to treat anxiety. In our study, yellow sugar cane brandy induced chronic anxiety in zebrafish after withdrawal on day 6, and abstinence was verified from day 8 to 10 without treatment with brandy (Figure 6). F-EtOAc (5.0mg/mL; i.p.) was shown to be effective in reversing anxiogenesis in adult zebrafish (Figure 7).
The present study showed the pharmacological potential of the ethanolic extract of Neem bark. Our findings demonstrated that the F-EtOAc, obtained after saponification of EtCNeem, showed to be rich in phenolic and flavonoid compounds with antioxidant potential, as well as a nontoxic, sedative and anxiolytic-like effect on adult zebrafish via the serotoninergic (5-HT1, 5-HT2A/2C and 5-HT3A/3B), GABAergic systems and anxiolytic effect in the chronic anxiety test. Thus, this study adds new evidence and highlights the potential of A. indica A. Juss for the development of plant-based remedies with anxiolytic properties.
We would like to thank the Master’s Degree Course in Natural Resources (MARENA) of the State University of Ceará. To our collaborators of the Research Group on Biotechnology of Natural Resources (BIOREN), as well as the State University of Ceará (UECE). We also would like to thank CAPES (National Coordination of Continuing Higher Education), CNPq (National Research and Development Council) and FUNCAP (State Foundation for Research Support of Ceará) for their support and scholarships.
The authors declare no conflicts of interest.

©2020 Silva, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.