eISSN: 2379-6367


Research Article Volume 8 Issue 3
1Department of Chemistry, Lomonosov Moscow State University, Russia
2Institute of Physiologically Active Compounds of RAS, Russia
Correspondence: Elena R Milaeva, Department of Chemistry, Lomonosov Moscow State University, 119991, Moscow, Leninskie gory, 1-3, Russia
Received: April 24, 2020 | Published: May 11, 2020
Citation: Nikitin EA, Shpakovsky DB, Pryakhin AD, et al. Antioxidant activity of modified 2,6-Di-tert-butylphenols with pyridine moiety. Pharm Pharmacol Int J. 2020;8(3):122-134. DOI: 10.15406/ppij.2020.08.00288
A series of 2,6-di-tert-butylphenols with pyridine moiety and their hydrophilic forms were synthesized and characterized by NMR, IR, X-ray and elemental analysis. The redox properties of compounds were studied by cyclic voltammetry (CV). Chemical oxidation of compounds under investigation leading to relatively stable radicals was studied by EPR. Antioxidant activity was evaluated in model reactions of hydrogen atom abstraction (DPPH-test) and one-electron reduction (CUPRAC-test). It was shown that modified 2,6-di-tert-butylphenols possess radical scavenging activity of prolonged action, achieving its maximum on 20 h time spans. The antioxidant properties in vitro in oxidation of linoleic acid by lipoxygenase, in reaction with superoxide radical-anion generated from xanthine/xanthine-oxidase enzymatic system, and in induced lipid peroxidation of rat liver homogenates were studied. The study of lipid peroxidation demonstrated high antioxidant activity for both lipophilic and hydrophilic compounds. The “structure-activity” analysis shows that the length of the linker between functional groups and position of substituent in pyridine cycle affects the antioxidant activity. Results of this study open the scopes for the search of novel water-soluble cyto-, neuroprotectors and physiologically active compounds with antioxidant mode of action.
Keywords: 2,6-di-tert-butylphenol, pyridines, antioxidants, X-ray analysis, DPPH, ROS, radical scavenging activity, electrochemistry, EPR, lipid peroxidation.
Oxidative stress is an imbalance between the action of antioxidants and pro-oxidants in favor of the latter.1,2 Pro-oxidants are generated in some cellular processes, including aerobic metabolism. Under certain conditions, pro-oxidants are produced at increased rate, thus violating the balance. Reactive oxygen species (ROS) such as superoxide radical-anion O2•¯, hydroxyl radical HO•, hydrogen peroxide H2O2, lipid hydroperoxides and peroxyl radicals are among the most well-known oxygen metabolites. ROS cause damage of intracellular structures such as proteins, nucleic acids, lipids, membranes by initializing destructive chain radical processes.3 It is well known that oxidative stress participates in a pathogenesis of many diseases, e.g. ischemia, atherosclerosis, inflammation,4 schizophrenia,5 pre-eclampsia,6 epilepsy,7 psoriasis,8 various types of cancer.9,10 Moreover, there exists the radical theory of ageing11,12 which assumes that endogenously produced ROS cause cumulative damage of living tissues due to disability of the organism to be entirely protected from active radicals. Considering this, the search of new methods of preventing such pathologies is a task of a great importance.
Antioxidants are the compounds which can neutralize the negative effect of the oxidative stress. They can be divided into several groups according to the mechanism of their action: first and the largest one includes substances that scavenge active radicals thus inhibiting chain radical processes; next group suppresses or prevents the formation of pro-oxidants; the last one repairs the damage caused by active particles.13 Living organism possesses complex antioxidant defense system which prevents cellular damage and regulates the toxic impact caused by ROS.14 It consists of low-molecular non-enzymatic natural antioxidants (e.g. ascorbic acid, tocopherols, lipoic acid, and carotenoids, etc.) and of enzymatic antioxidants such as superoxide dismutase (SOD), catalase, glutathione and glutathione dependent enzymes as well. These compounds demonstrate different mechanisms of activity: a dismutation of superoxide radical-anion by SOD, scavenging of hydroxyl radical HO•, interaction either with peroxyl radicals of lipids or lipid hydroperoxides breaking down the chain radical processes of lipid peroxidation.15 Thus, these natural antioxidants act in accordance with their chemical reactivity providing the non-harmful products.
Phenol derivatives (e.g. tocopherols, tyrosine, thyroxine, etc.) involved in radical biochemical processes are essential for cellular homeostasis. 2,6-Di-alkylphenols are less toxic than their unsubstituted analogues and often used as antioxidants, radical scavengers and biomimetics of α-tocopherol. The mechanism of their action includes formation of stable phenoxyl radicals and inhibition of further radical chain reactions.16 In last decade we focused on the synthesis of functionalized 2,6-di-tert-butylphenols and their metal complexes. An approach to combine antioxidant fragment with different metals in one molecule seems promising. Metalloporphyrins with 2,6-di-tert-butylphenol groups demonstrated dependence of the antioxidant properties on the nature of the metal.17 This provides multiple advantages: chemical structure and chemical properties may easily be altered by changing of metal, intermolecular redox process stabilizes phenoxyl radicals and terminal group can be introduced in order to endow the molecule with special properties, e.g. hydro/lipophilicity or affinity. Such compounds demonstrated wide spectrum of properties including antioxidant18-21 radical scavenging22,23 and cytotoxic properties24,25 that lets one to consider them as promising drug candidates.26,27
The goal of this study was the synthesis and evaluation of antioxidant activity of hybrid compounds containing both antioxidant moiety and coordination group capable to bind metal ions. A series of new pyridine derivatives containing 2,6-di-tert-butylphenol fragments (Scheme 1) was synthesized and characterized by physicochemical methods. Redox and antioxidant properties of compounds were evaluated by cyclic voltammetry and in model reactions with stable 2,2-diphenyl-1-picrylhydrazyl radical (DPPH), CUPRAC-test, enzymatic reactions of lipoxygenase (LOX 1-B) and xanthine oxidase. Additionally, compounds were studied in lipid peroxidation of rat liver homogenates ex vivo.
2,6-di-tert-butylphenol (99%), 3,5-di-tert-butyl-4-hydroxybenzoic acid (98%), triethylamine (≥99%), 2-picolylanime (99%), 3-picolylamine (≥99%) and 4-picolylamine (98%) were provided by Sigma-Aldrich and used with no further purification. 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid.28 The solvents (EtOH (95%), CHCl3, CH2Cl2, MeOH, Et2O, toluene, acetone, and petroleum ether (b.p. 40-70oC)) were used as-received.
Chlorides of 3,5-di-tert-butyl-4-hydroxybenzoic acid and 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid were synthesized by refluxion of acids with (10 eqv.) thionyl chloride in petroleum ether for 1 h followed by the removal of solvent in vacuo.
The NMR spectra were measured on a Bruker AMX-400 spectrometer in CDCl3 and DMSO-d6 (1H, 400MHz; 13C, 100.6MHz). The IR spectra were recorded on an IR200 Fourier-transform IR spectrophotometer (Thermo Nicolet) as KBr pellets. EPR spectra were recorded with a Bruker EMX spectrometer at the X-band range (9.8GHz). The measurements were carried out after pre-evacuation of samples solutions (concentration 0.1mM). The oxidant PbO2 was taken in a tenfold excess.
General synthesis of substituted amides (1-6) and their hydrochlorides (1a-6a)
A mixture of NEt3 (77 μl, 0.55 mmol) and corresponding picolylamine (52 μl, 0.5 mmol) in degassed CH2Cl2 (1 ml) was added dropwise to a solution of 3,5-di-tert-butyl-4-hydroxybenzoic acid chloride (137 mg, 0.5 mmol) or 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid chloride (148 mg, 0.5 mmol) in degassed CH2Cl2 (20 ml) and reaction mixture was stirred for 24 h. Solvent was removed in vacuo, residue was washed by petroleum ether (3x5 ml) and H2O (3x5 ml) and dried in the air.
3,5-di-tert-butyl-4-hydroxy-N-(pyridine-2-yl-methyl)benzamide (1)
Compound 1 was recrystallized from CH2Cl2-petroleum ether to give 133 mg of colorless powder (78%). Mp 170-172оС. IR, cm–1: 3345.9 (ν О-Н bound); 3332.5 (ν N-Н); 2873.4-2966.0 (ν С-Н); 1633.4 (ν С=О); 1523.5; 1434.8; 1322.9; 1234.2; 757.9. 1Н NMR (400 MHz, CDCl3, δ, ppm): 1.41 (s, 18H, 2 But); 4.55 (d, 2H, CH2, 3JHH=6 Hz); 7.25 (dd, 1H, H-Py, 3JHH=5 Hz, 3JHH=4 Hz); 7.30 (d, 1Н, Н-Py, 3JHH=4 Hz); 7.70 (s, 2H, H-Ar); 7.74 (dd, 1H, H-Py, 3JHH=4 Hz, 3JHH=5 Hz); 8.50 (d, 1H, H-Py, 3JHH=4 Hz); 8.96 (t, 1H, NH, 3JHH=6 Hz). 13C NMR (100.6 MHz, CDCl3, δ, ppm): 30.66 (C(CH3)3); 35.07 (C(CH3)3); 45.16 (NHCH2Py); 121.38 (C Py); 122.42 (C Py); 124.62 (C2 Ar); 125.69 (C1 Ar); 137.11 (C Py); 138.71 (C3 Ar); 149.19 (C Py); 157.28 (C4 Ar); 159.77 (C Py); 167.40 (C=O). Elemental analysis, calcd for C21H28N2O2 (%): С, 74.08; Н, 8.29; N, 8.23. Found (%): С, 73.89; Н, 8.60; N, 8.49.
3,5-di-tert-butyl-4-hydroxy-N-(pyridine-3-yl-methyl)benzamide (2)
Compound 2 was recrystallized from CH2Cl2-petroleum ether to give 153 mg of colorless powder (90%). Mp 108-110оС. IR, cm–1: 3627.5 (ν О-Н free); 3224.4 (ν N-H); 3060.5-2867.6 (ν С-Н); 1629.6 (ν С=О); 1598.7; 1546.6; 1430.9; 1363.4; 1326.8; 1224.6; 711.6. 1Н NMR (400 MHz, CDCl3, δ, ppm): 1.46 (s, 18H, 2 But); 4.67 (d, 2H, CH2, 3JHH=6 Hz); 5.62 (s, 1H, OH); 6.70 (t, 1H, NH, 3JHH=6 Hz); 7.27 (dd, 1H, H-Py, 3JHH=6 Hz, 3JHH=8 Hz); 7.65 (s, 2H, H-Ar); 7.73 (d, 1H, H-Py, 3JHH=8 Hz); 8.53 (d, 1H, H-Py, 3JHH=6 Hz); 8.59 (s, 1H, H-Py). 13C NMR (100.6 MHz, CDCl3, δ, ppm): 30.12 (C(CH3)3); 34.42 (C(CH3)3); 41.39 (CONHCH2Py); 123.62 (C Py); 124.23 (C2-Ar); 125.05 (C1-Ar); 134.51 (C Py); 135.78 (C Py); 136.02 (C3-Ar); 148.79 (C Py); 149.18 (C Py); 157.07 (C4-Ar); 168.21 (C=O). Elemental analysis, calcd for C21H28N2O2 (%): С, 74.08; Н, 8.29; N, 8.23; Found (%): С, 74.04; Н, 7.97; N, 8.18;
3,5-di-tert-butyl-4-hydroxy-N-(pyridine-4-yl-methyl)benzamide (3)
Compound 3 was recrystallized from CH2Cl2-petroleum ether to give 147 mg of colorless powder (86%). Mp 210-213оС. IR, cm–1: 3667.9 (ν О-Н free); 3232.1 (ν N-H); 3064.3-2875.3 (ν С-Н); 1631.5 (ν С=О); 1602.6; 1540.8; 1425.1; 1373.1; 1321.0; 1257.4; 1097.3; 740.5. 1Н NMR (400 MHz, DMSO-d6, δ, ppm): 1.41 (s, 18H, 2 But); 4.47 (d, 2H, CH2, 3JHH=6 Hz); 7.29 (d, 2H, H-Py, 3JHH=6 Hz); 7.45 (s, 1Н, ОН); 7.69 (s, 2H, H-Ar); 8.50 (d, 2H, H-Py, 3JHH=6 Hz); 8.94 (t, 1H, NH, 3JHH=6 Hz). 13C NMR (100.6 MHz, DMSO-d6, δ, ppm): 30.65 (C(CH3)3); 35.07 (C(CH3)3); 42.18 (CONHCH2Py); 122.61 (C Py); 124.61 (C2-Ar); 125.48 (C1-Ar); 138.74 (C3-Ar); 149.56 (C Py); 149.94 (C Py); 157.37 (C4-Ar); 167.48 (C=O). Elemental analysis, calcd for C21H28N2O2∙0.5 H2O (%): С, 72.17; Н, 8.36; N, 8.02. Found (%): С, 71.87; Н, 8.02; N, 8.15.
3-(3,5-di-tert-butyl-4-hydroxyphenyl)-N-(pyridine-2-yl-methyl)propanamide (4)
Compound 4 was recrystallized from CH2Cl2-petroleum ether to give 83 mg of colorless crystals (45%). Mp 127-130оС. IR, cm–1: 3100-3270 (ν О-Н bound, ν N-H); 3036.9-2866.7 (ν С-Н); 1633.4 (ν С=О); 1544.2; 1478.6; 1439.6; 1360.1; 1197.1; 1156.1; 878.4; 771.4; 749.7. 1Н NMR (400 MHz, CDCl3, δ, ppm): 1.43 (s, 18H, 2 But); 2.59 (t, 2H, CH2, 3JHH=8 Hz); 2.95 (t, 2H, CH2, 3JHH=8 Hz); 4.59 (d, 2H, CH2, 3JHH=5 Hz); 5.09 (s, 1H, OH); 6.71 (br. s, 1H, NH); 7.04 (s, 2H, H-Ar); 7.19-7.22 (m, 2H, 2 H-Py); 7.64-7.69 (m, 1H, H-Py); 8.54 (d, 1H, H-Py, 3JHH=5 Hz). NMR 13C (100.6 MHz, CDCl3, δ, ppm): 33.23 (C(CH3)3); 34.70 (ArCH2CH2CO); 37.22 (C(CH3)3); 41.91 (ArCH2CH2CO); 47.39 (CONHCH2Py); 124.99 (C2-Ar); 125.25 (C1-Ar); 127.75 (C Py); 132.74 (C Py); 134.35 (C Py); 138.86 (C3-Ar); 139.70 (C Py); 151.89 (C Py); 159.30 (C4-Ar); 175.44 (C=O). Elemental analysis, calcd for C23H32N2O2 (%): С, 74.96; Н, 8.75; N, 7.60; Found (%): С, 75.37; Н, 8.89; N, 7.43.
3-(3,5-di-tert-butyl-4-hydroxyphenyl)-N-(pyridine-3-yl-methyl)propanamide (5)
Compound 5 was recrystallized from CH2Cl2-petroleum ether to give 140 mg of colorless crystals (76%). Mp 145-147оС. IR, cm–1: 3565.7 (ν О-Н bound); 3249.5 (ν N-H); 3075.9-2867.6 (ν С-Н); 1643.1 (ν С=О); 1556.3; 1483.0; 1429.0; 1359.6; 1268.9 1238.1; 1101.2; 1039.4; 887.1; 869.7; 752.1; 719.3. 1Н NMR (400 MHz, CDCl3, δ, ppm): 1.43 (s, 18H, 2 But); 2.54 (t, 2H, CH2, 3JHH=8 Hz); 2.93 (t, 2H, CH2, 3JHH=8 Hz); 4.45 (d, 2H, CH2, 3JHH=6 Hz); 5.17 (s, 1H, ОН); 5.99 (t, 1H, NH, 3JHH=6 Hz); 7.01 (s, 2H, H-Ar); 7.24 (dd, 1Н, Н-Py, 3JHH=8 Hz, 3JHH=4 Hz); 7.49 (d, 1H, H-Py, 3JHH=8 Hz); 8.45 (d, 1H, H-Py, 3JHH=4 Hz); 8.49 (s, 1H, H-Py). NMR 13C (100.6 MHz, CDCl3, δ, ppm): 30.32 (C(CH3)3); 31.69 (ArCH2CH2CO); 34.32 (C(CH3)3); 38.92 (ArCH2CH2CO); 40.95 (CONHCH2Py); 123.65 (C1-Ar); 124.84 (C2-Ar); 131.14 (C Py); 135.54 (C Py); 136.14 (C3-Ar); 148.39 (C Py); 148.83 (C Py); 149.07 (C Py); 152.26 (C4-Ar); 175.44 (C=O). Elemental analysis, calcd for C23H32N2O2 (%): С, 74.96; Н, 8.75; N, 7.60. Found (%): С, 75.12; Н, 9.02; N, 7.84.
3-(3,5-di-tert-butyl-4-hydroxyphenyl)-N-(pyridine-4-yl-methyl)propanamide (6)
Compound 6 was recrystallized from CH2Cl2-petroleum ether to give 144 mg of colorless crystals (78%). Mp 163-165оС. IR, cm–1: 3253.8-3205.6 (ν О-Н bound, ν N-H); 3070.6-2871.0 (ν С-Н); 1636.8 (ν С=О); 1606.9; 1562.5; 1431.4; 1417.9; 1359.6; 1235.7; 1219.8; 1167.2; 1110.8; 1067.9; 1000.4; 885.2; 870.2; 793.6; 753.6; 697.6. 1Н NMR (400 MHz, CDCl3, δ, ppm): 1.43 (s, 18H, 2 But); 2.59 (t, 2H, CH2, 3JHH=8 Hz); 2.95 (t, 2H, CH2, 3JHH=8 Hz); 4.45 (d, 2H, CH2, 3JHH=6 Hz); 5.16 (s, 1H, ОН); 5.85 (t, 1H, NH, 3JHH=6 Hz); 7.02 (d, 2Н, СН-Py, 3JHH=6 Hz); 7.03 (s, 2H, H-Ar); 8.50 (d, 2H, H-Py, 3JHH=6 Hz). NMR 13C (100.6 MHz, CDCl3, δ, ppm): 30.32 (C(CH3)3); 31.66 (ArCH2CH2CO); 34.34 (C(CH3)3); 38.80 (ArCH2CH2CO); 45.16 (CONHCH2Py); 122.10 (C Py); 124.88 (C2-Ar); 131.00 (C1-Ar); 136.12 (C3-Ar); 147.41 (C Py); 149.98 (C Py); 152.31 (C4-Ar); 172.59 (C=O). Elemental analysis, for C23H32N2O2 calcd (%): С, 74.96; Н, 8.75; N, 7.60. Found (%): С, 74.18; Н, 8.78; N, 7.83.
General synthesis of hydrochlorides (1a-6a)
To a corresponding amide (0.1 mmol) in methanol (2 ml), conc. HCl (35%, 17 μl, 0.2 mmol) was added and stirred for 5 min. Then toluene (2 ml) was added with stirring for 5 min more. Solvents were removed in vacuo and then toluene (1 ml), methanol (1 ml) and chloroform (1 ml) were successively added and removed in vacuo. This operation was repeated twice, yield was quantitative.
3,5-di-tert-butyl-4-hydroxy-N-(pyridine-2-yl-methyl)benzamide hydrochloride (1a)
37 mg (100%) of yellowish powder of compound 1a was obtained. Mp 185-190 оС. IR, cm–1: 3628.4 (ν О-Н free); 3388.6 (ν N-H); 3062.9-2874.4 (ν С-Н); 1622.3 (ν С=О); 1539.9; 1470.0; 1434.3; 1364.9; 1317.1; 1241.0 1161.9; 1121.9; 768.0. NMR 1Н, (400 MHz, CDCl3, δ, ppm): 1.47 (s, 18H, 2 But); 5.04 (br.s, 2H, CH2) 5.62 (s, 1H, OH); 7.85 (s, 2H, Ar); 8.09 (br.s, 1H, Py); 8.37 (br.s, 1H, Py); 8.66 (br.s, 1H, Py); 8.87 (br.s, 1H, Py). NMR 13C (100.6 MHz, CDCl3, δ, ppm): 30.27 (C(CH3)3); 34.50 (C(CH3)3); 41.31 (CONHCH2Py); 123.29 (C Py); 125.14 (C2-Ar); 125.37 (C1-Ar); 127.94 (C Py); 135.95 (C3-Ar); 141.12 (C Py); 145.83 (C Py); 154.56 (C Py); 157.55 (C4-Ar); 168.13 (C=O). Elemental analysis, calcd for C21H28N2O2∙HCl (%): С, 66.92; Н, 7.75; N, 7.43. Found (%): С, 66.70; Н, 7.11; N, 7.05.
3,5-di-tert-butyl-4-hydroxy-N-(pyridine-3-yl-methyl)benzamide hydrochloride (2a)
37 mg (100%) of colorless powder of compound 2a was obtained. Mp 198-201оС. IR, cm–1: 3623.6 (ν О-Н free); 3216.7 (ν N-H); 3055.7-2877.8 (ν С-Н); 1629.6 (ν С=О); 1600.2; 1537.0; 1465.2; 1432.9; 1329.7; 1255.9; 1235.2; 1030.8; 888.1; 753.1; 681.7. NMR 1Н, (400 MHz, DMSO-d6, δ, ppm): 1.38 (s, 18H, 2 But); 4.60 (s, 2H, CH2); 7.67 (s, 2H, Ar); 8.00 (dd, 1H, Py, 3JHH=6 Hz); 8.50 (d, 1H, Py, 3JHH=8 Hz); 8.81 (d, 1H, Py, 3JHH=6 Hz); 8.87 (s, 1H, Py). NMR 13C (100.6 MHz, DMSO-d6, δ, ppm): 30.21 (C(CH3)3); 34.64 (C(CH3)3); 45.26 (CONHCH2Py); 124.31 (C2-Ar); 124.53 (C1-Ar); 126.87 (C Py); 138.35 (C3-Ar); 140.06 (C Py); 140.37 (C Py); 140.72 (C Py); 144.46 (C Py); 156.99 (C4-Ar); 167.11 (C=O). Elemental analysis, calcd for C21H28N2O2∙HCl (%): С, 66.92; Н, 7.75; N, 7.43. Found (%): С, 66.61; Н, 7.01; N, 7.52.
3,5-di-tert-butyl-4-hydroxy-N-(pyridine-4-yl-methyl)benzamide hydrochloride (3a)
37 mg (100%) of yellowish powder of compound 3a was obtained. Mp 260-263оС. IR, cm–1: 3388.8-3233.6 (ν О-Н bound; ν N-H); 3059.0-2872.5 (ν С-Н); 1631.5 (ν С=О); 1604.0; 1544.2; 1473.8; 1427.6; 1321.5; 1298.8; 1257.4; 1095.9; 905.9; 739.1. NMR 1Н, (400 MHz, CDCl3, δ, ppm): 1.40 (s, 18H, CH3); 4.81 (br.s, 2H, CH2) 5.65 (s, 1H, OH); 7.81 (s, 2H, Ph); 7.93 (br.s, 2H, Py); 8.56 (br.s, 2H, Py); 8.84 (br.s, 1H, NH). NMR 13C (100.6 MHz, CDCl3, δ, ppm): 29.81 (C(CH3)3); 34.07(C(CH3)3); 45.53(CONHCH2Py); 123.36(C Py); 124.64(C2-Ar); 125.37(C1-Ar); 135.69 (C Py); 139.82 (C3-Ar); 157.08 (C Py); 161.43 (C4-Ar); 168.21 (C=O). Elemental analysis, calcd for C21H28N2O2∙HCl (%): С, 66.92; Н, 7.75; N, 7.43. Found (%): С, 66.02; Н, 7.87; N, 7.23.
3-(3,5-di-tert-butyl-4-hydroxyphenyl)-N-(pyridine-2-yl-methyl)propanamide hydrochloride (4a)
40 mg (100%) of colorless powder of compound 4a was obtained. Mp 230-235оС. IR, cm–1: 3638.1 (ν О-Н free); 3256.7 (ν N-H); 3056.1-2871.5 (ν С-Н); 1662.8 (ν С=О); 1620.4; 1539.9; 1469.0; 1434.8; 1362.5; 1233.3; 1120.4; 1035.6; 767.5. NMR 1Н, (400 MHz, CDCl3, δ, ppm): 1.43 (s, 18H, CH3); 2.80 (br.s, 2H, CH2); 2.88 (br.s, 2H, CH2); 4.85 (br.s, 2H, CH2); 7.00 (s, 2H, Ph); 7.76 (br.s, 1H, Py); 7.84 (s, 1H, Py); 8.29 (br.t, 1H, Py); 8.55 (br.s, 1H, Py). NMR 13C (100.6 MHz, CDCl3, δ, ppm): 30.34 (C(CH3)3); 31.31 (ArCH2CH2CONH); 34.30 (C(CH3)3); 38.33 (ArCH2CH2CONH); 42.24 (CONHCH2Py); 124.90 (C2-Ar); 125.46 (C1-Ar); 129.19 (C Py); 131.04 (C Py); 135.71 (C Py); 138.95 (C3-Ar); 146.67 (C Py); 152.11 (C Py); 158.17 (C4-Ar); 173.52 (C=O). Elemental analysis, calcd for C23H32N2O2∙HCl (%): С, 68.21; Н, 8.21; N, 6.92. Found (%): С, 68.25; Н, 8.31; N, 6.87.
3-(3,5-di-tert-butyl-4-hydroxyphenyl)-N-(pyridine-3-yl-methyl)propanamide hydrochloride (5a)
40 mg (100%) of colorless powder of compound 5a was obtained. Mp 270-274оС. IR, cm–1: 3640.5 (ν О-Н free); 3250.00 (ν N-H); 3073.5-2870.5 (ν С-Н); 1635.8 (ν С=О); 1558.7; 1539.9; 1475.7; 1435.3; 1365.8; 1271.8; 1236.2; 1121.4; 1034.1; 786.8; 683.2. NMR 1Н, (400 MHz, DMSO-d6, δ, ppm): 1.33 (s, 18H, 2 But); 2.43 (t, 2H, CH2,3JHH=8 Hz); 2.72 ((t, 2H, CH2,3JHH=8 Hz); 4.44 (d, 2H, CH2, 3JHH=4 Hz); 6.90 (s, 2H, Ph); 7.94 (br.t, 1H, Py, 3JHH=8 Hz); 8.23 (br.d, 1H, Py, 3JHH=8 Hz); 8.75 (br.t, 1H, Py, 3JHH=8 Hz); 8.80 (br.s, 1H, Py). NMR 13C (100.6 MHz, DMSO-d6, δ, ppm): 30.47 (C(CH3)3); 31.04 (ArCH2CH2CO); 34.50 (C(CH3)3); 37.32 (ArCH2CH2CO); 45.28 (CONHCH2Py); 124.25 (C2-Ar); 125.35 (C1-Ar); 132.00 (C Py); 139.24 (C3-Ar); 139.65 (C Py); 140.40 (C Py); 140.61 (C Py); 144.01 (C Py); 151.96 (C4-Ar); 172.39 (C=O). Elemental analysis, calcd for C23H32N2O2∙HCl (%): С, 68.21; Н, 8.21; N, 6.92. Found (%): С, 68.70; Н, 8.13; N, 6.68.
3-(3,5-di-tert-butyl-4-hydroxyphenyl)-N-(pyridine-4-yl-methyl)propanamide hydrochloride (6a)
40 mg (100%) of yellowish powder of compound 6a was obtained. Mp 230-240оС. IR, cm–1: 3618.8 (ν О-Н free); 3261.0 (ν N-H); 3062.4-2871.5 (ν С-Н); 1669.6 (ν С=О); 1598.7; 1544.2; 1505.7; 1435.3; 1370.2; 1313.3; 1230.4; 1171.1; 1029.3; 874.6; 790.7. NMR 1Н, (400 MHz, CDCl3, δ, ppm): 1.38 (s, 18H, 2 But); 2.69 (br.s, 2H, CH2); 2.93 (br.s, 2H, CH2); 4.64 (br.s, 2H, CH2); 7.03 (s, 2H, Ph); 7.67 (br.s, 2H, Py); 8.57 (br.s, 2H, Py). NMR 13C (100.6 MHz, CDCl3, δ, ppm): 30.40 (C(CH3)3); 31.27 (ArCH2CH2CO); 34.35 (C(CH3)3); 38.26 (ArCH2CH2CO); 42.58 (CONHCH2Py); 125.08 (C2-Ar); 125.38 (C1-Ar); 130.31 (C Py); 136.24 (C3-Ar); 143.95 (C Py); 150.26 (C Py); 152.16 (C4-Ar); 172.36 (C=O). Elemental analysis, calcd for C23H32N2O2∙HCl (%): С, 68.21; Н, 8.21; N, 6.92. Found (%): С, 68.16; Н, 8.16; N, 6.90.
Crystallographic data collection and structure determination
All diffraction data were collected on a STOE StadiVari Pilatus 100 K diffractometer λ(MoKα) = 0.71073 Å, λ(CuKα) = 1.5418 Å, ω-scans at 293 K.29 The primary processing of the experimental data array was performed using the WinGX program package.30 The structures were solved by direct methods and refined by full-matrix least-squares procedures on F2 using SHELXL97.31 All non-hydrogen atoms were refined anisotropically, and hydrogen atoms were located at calculated positions and refined via the “riding model”. Crystal data and structure refinement parameters are listed in Table 1. CCDC 1960176 (4), 1960175 (5), 1960174 (6) contain the supplementary crystallographic data for this paper. The structures of complexes were drawn using the MERCURY CSD 3.1 program.32
|
Compound |
4 |
5 |
6 |
|
Empirical formula |
C23H32N2O2 |
C23H32N2O2 |
C27H32N2O2 |
|
Fw |
368.5 |
368.5 |
416.54 |
|
Temperature (K) |
293(2) |
293(2) |
293(2) |
|
Space group |
P21/c |
P21/c |
P21/c |
|
Syngony |
Monoclinic |
Monoclinic |
Monoclinic |
|
a (Å) |
16.3269(3) |
13.4955(5) |
18.2222(3) |
|
b (Å) |
8.6138(3) |
16.9419(5) |
14.1002(2) |
|
c (Å) |
16.3350(4) |
9.7897(4) |
17.5282(3) |
|
β (°) |
104.667(3) |
105.059(3) |
97.235(2) |
|
V (Å3) |
2222.44(11) |
2161.44(14) |
4467.78(13) |
|
Z |
4 |
4 |
8 |
|
Drmax/Drmin (e/Å3) |
0.226/-0.215 |
0.235/-0.169 |
0.263/-0.248 |
|
λ |
CuKα |
CuKα |
CuKα |
|
μ (mm−1) |
0.548 |
0.563 |
0.609 |
|
R1/wR2 (I ≥ 2σ(I) |
0.0546/0.1336 |
0.0573/0.1490 |
0.0490/0.1273 |
|
GOOF |
0.824 |
1.004 |
0.941 |
Table 1 Crystal data and the structure refinement details for compounds 4-6
Electrochemical study
All measurements were carried out under argon at room temperature. Cyclic voltammetry experiments were performed in classical three-electrode cell in CH3CN solution with 0.05M Bu4NBF4 as supporting electrolyte using a IPC-Win potentiostat. The number of electrons transferred was determined by comparing with the height of Fc2+/Fc3+ wave for the same concentration and by rotating disk electrode method as well. A platinum or glassycarbon (GC) working electrode with diameter 2mm, platinum wire auxiliary electrode and aqueous Ag/AgCl/KCl (sat.) reference electrode were used. The CH3CN prior to use was dried over CaH2 and distilled over P2O5.
Antioxidant assay
Electrochemical DPPH test
Antioxidant activity of compounds 1-6 and 1a-6a was studied in the reaction with stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) in МeCN using CV method.33,34 The rate of the process was monitored by the change of the DPPH reduction current intensity (with the ratio of concentrations of compounds and DPPH being 1:1 and 1:2 (C0=10–3 mol*L–1). The CVA curves were recorded after certain periods of time (30 s, 1 and 3 min) at 100 mV/s scan rate. The values of antioxidative activity A=(1–C/C0)•100(%) of compounds under study were determined for the moment of reaching steady state of reaction ("plateau"). The values of I0, obtained by the equation of a calibrating curve at a given concentration of DPPH were used for the plotting the kinetic curves and determining the stoichiometry values.
Spectrophotometrical DPPH assay
The radical scavenging activity was evaluated using the radical 2,2-diphenyl-1-picrylhydrazyl (Sigma-Aldrich) by spectrophotometry at λmax = 517 nm according the known procedure.35 The reaction was performed in 1cm glass cuvettes. The reaction mixture contained DPPH (0.75 ml, 0.2mM) and a solution of the test compound in EtOH (0.75 ml, 0.2mM). The reaction was monitored for 20 h. The data were calculated using the Microsoft Excel 2010. The antioxidant activity was calculated as percentage of reduced DPPH according to the formula I (%)= (A0−A1)/A0•100, where A0 is the absorbance of the control 0.1mM DPPH in EtOH, ε DPPH = 1.15 104, A1 is the absorbance of the reaction mixture in the presence of the test compound.
CUPRAC assay
The ability of the compounds to one-electron transition was measured by means of reduction of complex of 2,9-dimethyl-1,10-phenanthroline (neocuproine, Sigma-Aldrich, 98%) with copper by spectrophotometry at λmax = 450 nm. The known procedure36 was modified for plate spectrophotometer. The reaction was performed in plate wells (96 wells). The volume of reaction mixture inside one well was 0.2 ml and consisted of 0.05 ml of ammonium acetate buffer (pH 7.0), 0.05 ml of 10mM CuCl2 solution in methanol, 0.05 ml of 7.5mM neocuproine solution in methanol and 0.05 of 0.5mM tested compound solution in methanol. The results were compared to Trolox and expressed in trolox equivalent antioxidant capacity (TEAC) and calculated according to the formula TEAC = A1/A0, where A1 is the absorbance of the reaction mixture in the presence of the tested compound and A0 is the absorbance of the solution of trolox of the same concentration.
NBT assay
The effects of compounds 1-6 and 1a-6a on enzymatic generation of the superoxide radical anion О2•– in the xanthine-xanthine oxidase system were estimated by the amount of reduced tetrazolium blue into formazan.37
The composition of the reaction mixture in 96 well plate was as follows: carbonate buffer (270 µl, 40mM, pH 10.0) containing EDTA (0.1mM), a solution of xanthine (6 µl, 10mM) in carbonate buffer, a 0.5% bovine serum albumin (3 µl) in water, nitro blue tetrazolium chloride (NBT, 3 µl, 2.5mM) in water, and a solution of the compound under study in DMSO (6 µl of 5mM concentration). Xanthine oxidase (12 µl, 0.004 units) in buffer was added to the mixture at rt and the absorption at 560 nm (λmax blue formazan) was recorded by Thermo Scientific Multiskan Go microplate spectrophotometer for 600 s. The control experiment was performed in the presence of DMSO (6 µl) without compound. All experiments were performed in triplicate.
Inhibition I (%) = (1 − Ai/A0) × 100%, where Ai is the absorbance in the presence of the testing compound at the end of the reaction (600 s), A0 is the absorbance of the blank solution.
Inhibition of lipoxygenase (LOX 1-B)
The lipoxygenase activity was evaluated spectrophotometrically.38 The concentrations of linoleic acid oxidation products, isomeric hydroperoxides, were measured at λmax = 234 nm (ε = 25000L mol–1 cm–1) with a 96-well microplate spectrophotometer Multiskan Go (Termo Scientific, USA). The analyzed solution contained 30 µl borate buffer (pH 9.0), 100 µl linoleic acid (0.45mM) in borate buffer, 3 µl 1mM solution of test compound in DMSO. The reaction was initiated by the addition of 17 µl of lipoxygenase (500 U) solution in borate buffer.
The measurements were performed for 5 min at 20°C.
The inhibition rate I (%) of lipoxygenase was determined by the formula:
I (%) = (ν0/ν0´)•100%,
where v0 and v0´ are initial rates of the enzymatic reaction in the presence and absence (control) of the compounds under study, respectively.
The initial rate (v0 and v0´) was calculated by the formula
ν0 = ΔС/Δt = ΔA/(Δt•ε) = tgα/(Δt•ε),
where A0 is the absorbance of the control solution, and A1 is the absorbance of the reaction mixture in the presence of the tested compound 5 min after the beginning of the reaction. All experiments were performed in triplicate.
Mitochondrial lipid peroxidation
Lipid peroxidation in mitochondrial suspension was followed by the accumulation of substances that reacted with thiobarbituric acid (TBARs), and monitored spectrophotometrically according to the procedure described previously.39 Mitochondrial membranes lipid peroxidation was induced by using FeNH4(SO4)2・12H2O (Fe3+; 0.5mM) or tert-butylhydroperoxide (tBHP) as an oxidizing agent.
Mitochondria preparation and assays
Mitochondria were isolated by conventional differential centrifugation from the livers of adult Wistar strain rats fasted overnight in 5mM HEPES buffer (pH 7.4) containing 210mM mannitol, 70mM sucrose, and 1mM EDTA.40 Since mannitol binds hydroxide ions, it was omitted during the final centrifugation and resuspension of mitochondria in experiments with lipid peroxidation estimation. The mitochondrial fraction contained 130-150 mg of protein per liver. Mitochondria were stored at 4oC. Functional activity of rat liver mitochondria remained constant during 3-4 h. Protein concentrations were determined by the biuret assay using bovine serum albumin as a standard.41 Mitochondrial potential and mitochondrial swelling were performed in accordance with our previous work.42
MTT assay
The toxicity of compounds was studied on primary cultures of rat brain cortex neurons with standard MTT assay.43,44
Chemistry and structural study
Amides 1-6 with various length of a linker and different position of a nitrogen atom in the pyridine ring were synthesized by the interaction of acids chlorides with different picolylamines in the presence of NEt3 for accepting of liberated HCl (Scheme 1). Reactions were carried out in CH2Cl2 under room temperature to give stable products with 45-90% yields as colorless powders. For the hydrophilization of compounds amides 1-6 were treated with 3 molar equivalents of conc. HCl in MeOH by previously described method45 to yield corresponding hydrochlorides 1a-6a after removing of solvent. Compounds obtained were stable in air and in solutions and were characterized by IR, 1H and 13C NMR spectroscopy and elemental analysis.
The recrystallization of compounds 4-6 from the mixture of CH2Cl2 - petroleum ether gave the monocrystals. The crystal and molecular structures of compounds 4-6 were determined by X-ray diffraction. The crystallographic characteristics and selected bond lengths and bond angles for compounds 4-6 are summarized in Tables 1, 2. Compounds 4 and 5 in the solid state were found to be monomers (Figure 1A, 1B). In the case of 4 the angle between the planes of benzene and pyridine rings is 84.08°. The intermolecular hydrogen bonds N2-H2 ⋯ N1 and O1-H1 ⋯ O2 with the distances H2 ⋯ N1 = 2.132 Å and H1 ⋯ O2 = 1.795 Å respectively were detected.
|
Bond lengths (Å) |
|||||
|
4 |
5 |
6 |
|||
|
O1–C1 |
1.382(2) |
O1–C1 |
1.227(2) |
O1–C1 |
1.3725(19) |
|
O1–H1 |
0.96(4) |
O2–C7 |
1.376(3) |
O1–H12 |
0.84(3) |
|
O2–C11 |
1.228(2) |
O2–H11 |
0.92(3) |
O2–C17 |
1.234(2) |
|
N1–C13 |
1.335(3) |
N1–C1 |
1.328(3) |
N1–C17 |
1.327(2) |
|
N1–C17 |
1.340(3) |
N1–C18 |
1.456(3) |
N1–C18 |
1.449(2) |
|
N2–C11 |
1.336(3) |
N1–H1 |
0.99(2) |
N1–H11 |
0.92(2) |
|
N2–C12 |
1.456(3) |
N2–C22 |
1.321(5) |
N2–C22 |
1.324(2) |
|
N2–H2 |
0.86(3) |
N2–C23 |
1.323(4) |
N2–C21 |
1.325(2) |
|
Angles (°) |
|||||
|
C1–O1–H1 |
109(2) |
C7–O2–H11 |
116(2) |
C1–O1–H12 |
118.1(18) |
|
C13–N1–C17 |
117.3(2) |
C1–N1–C18 |
122.30(19) |
C17–N1–C18 |
121.87(15) |
|
C11–N2–C12 |
121.8(2) |
C1–N1–H1 |
119.0(13) |
C17–N1–H11 |
120.5(13) |
|
C11–N2–H2 |
120.3(16) |
C18–N1–H1 |
118.7(13) |
C18–N1–H11 |
117.1(13) |
|
C12–N2–H2 |
117.8(16) |
C22–N2–C23 |
116.6(3) |
C22–N2–C21 |
115.85(15) |
|
O2–C11–N2 |
120.9(2) |
O1–C1–N1 |
122.5(2) |
O2–C17–N1 |
121.97(16) |
Table 2 Selected bond lengths (Å) and angles (°) for compounds 4-6
In the structure of 5 the angle between the planes of benzene and pyridine rings was 19.61°. The intermolecular hydrogen bonds N1-H1 ⋯ O1 and O2-H11 ⋯ N2 with the distances H1 ⋯ O1 = 1.813 Å and H11 ⋯ N2 = 2.234 Å respectively were found.
Compound 6 appeared to be a homodimer (Figure 1C). Two monomers are arranged in such a way that pyridine ring of one molecule is located opposite to the hindered phenol fragment of another one resulting in the formation of hydrogen bonds O1-H12 ⋯ N2A and O1A-H11A ⋯ N2 with the distances H12 ⋯ N2A = 2.082 Å and H11A ⋯ N2 = 1.998 Å respectively. Also, the intermolecular hydrogen bonds N1-H11 ⋯ O2A and N1A-H12A ⋯ O2 with the distances H11 ⋯ O2A = 1.911 Å and H12A ⋯ O2 = 1.865 Å respectively were revealed.
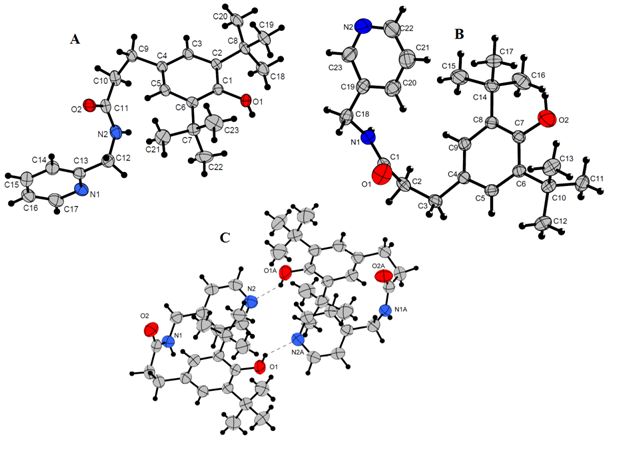
Figure 1 Molecular structures of compounds 4 (A), 5 (B), 6 (C). Thermal displacement ellipsoids are given at the 50% probability level. Hydrogen atoms are omitted for clarity.
Formation of phenoxyl radicals
Phenoxyl radicals generated by the oxidation of corresponding para-substituted di-tert-butyl phenols were described previously.46 Stability of radicals was influenced by electron-donor and electron-withdrawing properties of para-substituents of phenol group.
The formation of radicals during chemical oxidation of compounds 1-6 (PbO2 in toluene) was proved by EPR spectroscopy. The X-band EPR spectra measured at 293 K show the spin density distribution in the organic ligand. The spectra of radicals 1-3 are triplets corresponding to the coupling of the unpaired electron with two equivalent meta-protons (1H, S = 1/2) of the phenoxyl ring while radicals derived from 4-6 demonstrate also hyperfine coupling constants of two equivalent protons of benzyl CH2 group (Figure 2). The parameters of the EPR spectra of radicals (Table 3) are close to those of similar phenoxyl radicals.22
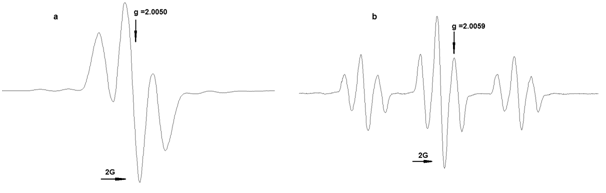
Figure 2 EPR spectra of radicals generated from compounds 2 (a) and 4 (b) by PbO2 in toluene (293 K).
|
Compound |
g-value |
а2H(Ar), mT |
а2H(ArCH2), mT |
|
1 |
20,054 |
0.18 |
- |
|
2 |
2.005 |
0.215 |
- |
|
3 |
2.0058 |
0.215 |
- |
|
4 |
2.0056 |
0.17 |
0.79 |
|
5 |
2.0053 |
0.17 |
0.78 |
|
6 |
2.0059 |
0.17 |
0.78 |
Table 3 The isotropic g-value and hyperfine coupling constants (a) for radicals 1-6
The radicals were stable in solutions at room temperature under anaerobic conditions. The intensity of radicals generated from 1-3 and bearing electron-withdrawing amide fragment in para-position which can participate in delocalization of unpaired electron is one order higher than that of radicals from 4-6 possessing electron-donor alkyl moiety. The similar effects have been observed for phenoxyl radicals containing transition metals.47
Electrochemical study
Voltammetric methods have often been applied for elucidation of activity mechanism of natural and synthetic phenolic antioxidants.48,49 In this work the redox properties of compounds 1-6 as well as hydrochlorides 1a-6a were measured and their electrochemical behavior was studied by cyclic voltammetry (CV) method using Pt working electrode. The redox potentials values are summarized in Table 4.
|
Compound |
Oxidation potential, V |
|
|
Еа1 |
Еа2 |
|
|
1 |
1.2 |
- |
|
2 |
1.27 |
- |
|
3 |
1.24 |
- |
|
4 |
1.23 |
1.57 |
|
5 |
1.05 |
1.42 |
|
6 |
- |
1.7 |
|
1а |
1.2 |
1.65 |
|
2а |
1.13 |
- |
|
3а |
1.2 |
1.7 |
|
4а |
1.14 |
- |
|
5а |
1.1 |
- |
|
6а |
0.98 |
1.6 |
|
BHT* |
1.63 |
- |
Table 4 The redox potentials values (Ea, V) of compounds 1-6 and 1a-6a (CH3CN, 5·10-2 М n-Bu4NBF4, Pt electrode, scan rate 100 mV/s, via Ag|AgCl|KCl(sat.))
*Butylated hydroxytoluene
The redox behavior of phenols under study strongly depends on the structure of substituent in para-position and the length of hydrocarbonyl linker. Compounds 1-3 shows wide one-electron oxidation peak on voltammogramm at potentials Ea = 1.2-1.27 V (Figure 3). The irreversibility of this wave points out the EC mechanism (electron transfer followed by the fast-chemical reaction). These data are in accordance with results obtained in50 concerning electrochemical behavior of acetanilide, propanyl and N,N-diphenylacetamide indicating the involvement of amide nitrogen atom in oxidation.
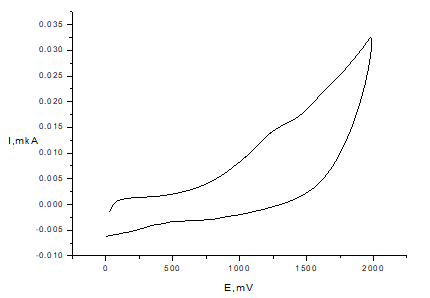
Figure 3 CV of compound 2 in anodic range (С=1mМ, Pt electrode, 50mМ TBABF4, CH3CN, scan rate 100 mV/s, vs. Ag/AgCl)).
At the same time, the introduction of -(CH2)2- linker in compounds 4 (Figure 4) and 5 leads to the marked change of electrochemical behavior: the two one-electron irreversible peaks of oxidation at the anodic region are displayed in the potential range 1.05-1.57 V.
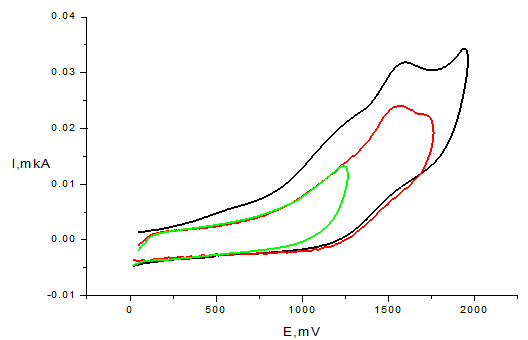
Figure 4 CV of compound 4 (С=1mМ, Pt electrode, 50mМ TBABF4, CH3CN, scan rate 100mV/s, vs. Ag/AgCl)).
The first peak on CV curves of compounds 4 and 5 (at less anodic potentials Ea = 0.96-1.30 V) can be associated with N-centered one-electron oxidation of amide group, resulting in formation of radical cation I (Scheme 2) and followed by fast deprotonation, as it was observed in case of ferulic and caffeic acid amides.51,52 The second peak at 1.42 – 1.57 V corresponds to further one-electron oxidation of methylene fragment that leads to the formation of N-acylium cation II.53
Cyclic voltammograms of compounds were also recorded at different sweep rates. Linear plots of peak current (Ip) as a function of the square root of scan rate (n) were obtained indicating that oxidation processes are diffusion controlled.54
However, compound 6 demonstrate different pattern of electrochemical activity by showing irreversible two-electron oxidation peak at Ea = 1.7 V (Figure 5). In the case of compounds 1a-6a the one-electron peak at 1.0-1.2 V appears as well what obviously corresponds to Cl-/Cl0 oxidation.55 The peak corresponding to oxidation of phenol moiety is observed only for compounds 1a, 3a and 6a (Figure 5) at more positive potentials than in case of corresponding phenols 1, 3, 6 (Table 4). This phenomenon may be due to the considerable shift of oxidation potential to anodic range because of electron-withdrawing influence of positively charged pyridinium fragment.
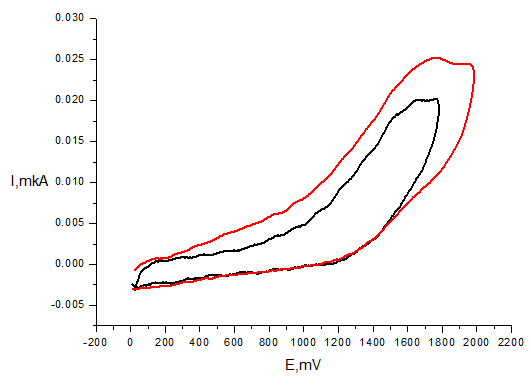
Figure 5 CV of compound 6 (С=1mМ, Pt electrode, 50mМ TBABF4, CH3CN, scan rate 100mV/s, vs. Ag/AgCl)).
Evaluation of antioxidant activity
The antioxidant properties of compounds 1-6 were studied in model reactions. Redox properties and radical scavenging ability were evaluated by cyclic voltammetry in 2,2-diphenyl-1-picrylhydrazyl (DPPH) test. Affinity to one-electron transfer was measured by CUPRAC-test. The ability to interact with superoxide radical anion was measured using the xanthine-xanthine oxidase system. The inhibition of oxidation of linoleic acid by lipoxygenase in vitro and rat liver lipid peroxidation ex vivo was estimated.
Electrochemical DPPH-test
Oxidation peaks for studied compounds are observed at more anodic region (Table 4) than that of 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) for which the first reduction wave appeared at peak potential 0.28 V.56 The cyclic voltammogram of DPPH (Figure 6) in CH3CN displays two one-electron reversible peaks that correspond to oxidation and reduction of this radical (Scheme 3).57
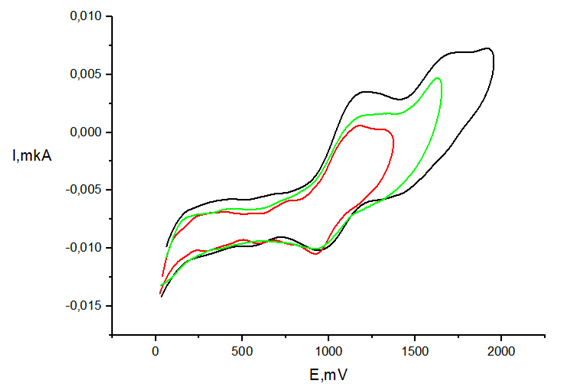
Figure 6 CV of compound 1a (С=1mМ, GC electrode, 50mМ TBABF4, CH3CN, scan rate 100mV/s, vs. Ag/AgCl)).
Therefore, CV method can be used in order to study antioxidant activity in reaction of H-transfer to DPPH. The fact that the redox potentials values of 1-6 and 1a-6a do not overlap with potentials of DPPH oxidation/reduction allows one to apply the CV method for estimation of antioxidant activity.33,34
In the presence of the antioxidants the current values of both peaks decreased (Figure 7).
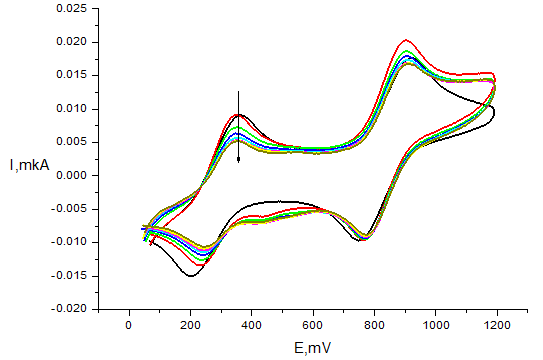
Figure 7 Voltammogram of 1mМ DPPH in the presence of 0.5mМ compound 6 on Pt electrode in СН3СN (v=100mV/s, 5mМ Bu4NBF4, time of reaction 30min, Ag|AgCl|KCl (sat.)).
According to the Randles-Shevchik equation at specific electrode surface area and potential scan rate the ratio of radical reduction and oxidation peak currents during the reaction is equal to the concentration ratio I/I0 = C/C0 (I0 is the value current without antioxidant, I is the current at a chosen time of reaction). Hence, we used this relation to quantitatively estimate the antioxidant efficiency (AOE) as the percentage of reacted DPPH:
AOE = (1−Ifin /I0) × 100 (%) = (1−Cfin /C0) × 100 (%),
where C0 is the starting DPPH concentration, Cfin is DPPH concentration in the end of the reaction.
This simple method has some advantages compared to spectrophotometrical test. Electrochemical test allows to use higher concentrations of antioxidants that leads to the increase of reaction rate with DPPH. The compounds 1-3 did not show any significant scavenging activity. Even at concentrations ratio DPPH/compound 1:1 (C = 1mM) the amount of reacted radical was about 6-9%. But in the case of 4-6 the activity changes dramatically. Thus at 1:1 ratio the amount of reduced DPPH was close to 100%. The compounds 4 and 5 demonstrated high activity: at DPPH/compound concentrations ratio 2:1 the amounts of DPPH quenched were 34% and 22% (at reaction time 30 min). After 48 h these amounts reached values of 67% and 58%, respectively, that points out the stoichiometry of reaction more than 1. According to these data compounds 4-6 are antioxidants with prolonged action effect. Therefore, the length of the linker between phenol and amide fragments strongly affects the radical scavenging activity of presented compounds.
The activity of compounds 1a-6a in electrochemical tests differs slightly from the activity of compounds 1-6. At concentrations ratio 1:1 the compounds 1a-3a very slowly react with DPPH (the amount of radical quenched is 3-5% even after 24 h of reaction proceeding). But the compounds 4a-6a demonstrated high activity: at equimolar concentrations DPPH reacted completely (AOE = 100% at reaction time 24 h). Thereby, the results of electrochemical test can be compared with data obtained spectrophotometrically as well.
Spectrophotometrical DPPH-test
DPPH is violet-colored stable radical with the maximum of absorbance at 517 nm while the 2,2-diphenyl-1-picrylhydrazine (DPPH-H) is pale yellow. This radical is a useful reagent for the investigation of the radical scavenging activity of phenols, catecholes, etc. The amount of reacted DPPH can be defined by method suggested by W. Brand-Williams.58 The accepted reaction mechanism at the first stage includes hydrogen atom abstraction from an antioxidant to give DPPH-H and corresponding radical followed by secondary transformations. The reaction of 0.1mM of synthesized compounds in ethanol with equimolar concentration of DPPH involves a color change from violet to yellow, which can be monitored spectrophotometrically by measuring the decrease in absorbance for 20 h at 517 nm (Figure 8).

Figure 8 Changes in UV-vis spectrum of DPPH (0.1mM) in the presence of 0.1mM compound 6 in EtOH, 20oC.
The percentage of reduced DPPH in the presence of test compounds was increased with time compared to control (0.1mM DPPH) at the same time spans (Table 5). The amount of reduced DPPH was lower for compounds 1-3 and 1a-3a than that of their analogues 4-6 and 4a-6a due to the difference in chemical structure of phenoxyl radicals which in the case of –(CH2CH2)-bearing radical can participate in further reactions with DPPH as it was demonstrated previously.43 It can be noticed that after 20 h of reaction the percentage of reduced DPPH by compounds was much higher for compounds 4-6, 4a-6a in comparison with control (Figure 9). The antioxidant abilities of tested compounds were compared to that of BHT.
|
Compound |
Time, h |
|||||||
|
0.5 |
1 |
1.5 |
2 |
2.5 |
3 |
5 |
20 |
|
|
1 |
12.7 |
14.3 |
15.5 |
16 |
17.2 |
17.8 |
18.5 |
36.6 |
|
2 |
1 |
3.6 |
5.3 |
6.6 |
8.3 |
9.2 |
12 |
18.9 |
|
3 |
3.3 |
4.9 |
6.1 |
7 |
8.1 |
9 |
11.8 |
13.3 |
|
4 |
6.6 |
18.8 |
27.8 |
35.3 |
41.9 |
47.1 |
70.8 |
87.7 |
|
5 |
13.6 |
29 |
40.3 |
48.7 |
55.7 |
61 |
77.1 |
89.4 |
|
6 |
7.6 |
14.4 |
20.1 |
25.3 |
30.3 |
34.9 |
50.4 |
90.9 |
|
1a |
7.9 |
9.7 |
11 |
12.4 |
13.7 |
15 |
17 |
21.6 |
|
2a |
10.5 |
11.9 |
13 |
14.9 |
15 |
16.6 |
16.6 |
22.5 |
|
3a |
1.4 |
3.5 |
5.4 |
6.3 |
7.9 |
9 |
13.1 |
25.4 |
|
4a |
9 |
13.2 |
17 |
20.5 |
23.7 |
26.9 |
34.7 |
62.4 |
|
5a |
7.7 |
11.8 |
15.7 |
19.5 |
23 |
26.4 |
37 |
73.3 |
|
6a |
11.1 |
15.1 |
18.9 |
22.2 |
25.4 |
28.4 |
38.1 |
69.2 |
|
BHT |
1.3 |
3.4 |
5.3 |
6.3 |
7.8 |
8.9 |
13.2 |
22.6 |
Table 5 The amount of reduced DPPH (%) for compounds 1-6 and 1a-6a
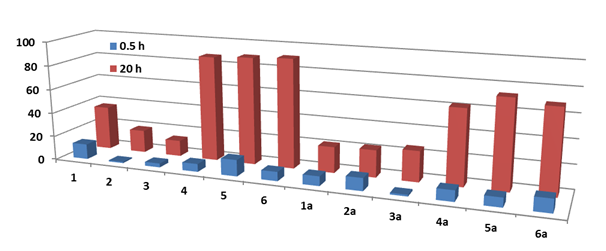
Figure 9 Comparison of the amount of reduced DPPH (%) in the presence of compounds 1-6 and 1a-6a on 0.5 h and 20 h.
In the case of –(CH2CH2)- linker such ability is restricted and the antioxidant activity of amides from phenol containing propionic acid was higher. To conclude the presence of the linker between phenol and amide fragments influences the anti-radical activity of compounds.
Thus, the data of electrochemical and spectrophotometrical DPPH tests correlate well enough (considering the difference of the reacting substances concentrations).
One-electron transfer capability (CUPRAC test)
Capability of the compounds to abstract one-electron makes them the potential antioxidants. The one-electron transfer capacity of compounds was examined as Cu2+ complex with 2,9-dimethyl-1,10-phenanthroline (neocuproine) reducing ability in method proposed by Apak et al. (Scheme 4).36 Antioxidant activity of compounds is compared to standard antioxidant trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) as an analogue of α-tocopherol. While Cu2+ complex is light blue the reduction with antioxidant changes its color to yellow orange with λmax at 450 nm that can be detected spectrophotometrically.
Results were presented in Trolox equivalents (TEAC–Trolox equivalent antioxidant capacity). The comparison of TEAC values allows one to conclude that the pyridine fragment plays a significant role in electron transfer to the Cu2+ ion (Table 6). The capability to one-electron transfer was higher for ortho-substituted pyridines.
|
Compound |
TEAC* |
NBT assay ** I (%) |
Inhibition of LOX 1B*** I (%) |
|
1/1a |
0.89 / 1.21 |
10.7 / 12.4 |
7.6/19.2 |
|
2/2a |
0.29 / 0.54 |
8.8 / 5.0 |
8.5/1.7 |
|
3/3a |
0.35 / 0.61 |
19.1 / 5.6 |
12.0/1.0 |
|
4/4a |
1.71 / 1.35 |
2.9 / 2.1 |
1.4/5.7 |
|
5/5a |
1.00 / 1.05 |
4.1 / 13.0 |
5.3/7.3 |
|
6/6a |
1.10 / 1.56 |
7.0 / 11.9 |
2.6/0.6 |
|
BHT |
1.1 |
10.3 |
50**** |
Table 6 TEAC in CUPRAC test; A (%)-reduced superoxide radical anion in percentage of control; I (%)-inhibition of linoleic acid oxidation by LOX 1-B for compounds 1-6 and 1a-6a
*-concentration of compound 0.5mM; ** - 5mM; *** - 1mM; ****-0.8mM
Superoxide radical anion scavenging activity (NBT assay)
The ability of tested compounds to reduce superoxide radical anion О2•– generated in enzymatic oxidation of xanthine by xanthine oxidase to uric acid was studied.37 The percentage of reduced radical anion was defined spectrophotometrically by detection of blue formazan formation (λmax 560 nm) from nitro blue tetrazolium (NBT). It was found that all the compounds are frugal reducers of О2•– with activity percentage within 2-19% range (Table 6).
Lipoxygenase inhibitory assessment
Lipoxygenases catalyze regio- and stereoselective oxidation of polyunsaturated fatty acids containing one or more (1Z,4Z) pentadiene fragments to the corresponding hydroperoxides.59 This reaction is the first step in the biosynthesis of leukotrienes - mediators of various inflammatory processes and allergic reactions participating in the pathogenesis of neoplastic diseases, asthma, and atherosclerosis.60 Moreover, the side products of this reaction are superoxide radical anion and hydrogen peroxide. Thereby, lipoxygenase is an important pharmaceutical target and many attempts have been made to find a selective lipoxygenase inhibitor.61,62 Soybean lipoxygenase 1-B (LOX 1-B) is frequently used as a model enzyme in the study of homological lipoxygenase family. In particular, 5-lipoxygenases acting in the human body and LOX-1-B catalyze oxidation of linoleic acid to isomeric hydroperoxyoctadienoic acids. Since tested compounds possess antioxidant properties they have been examined as LOX 1-B inhibitors.
The reaction of linoleic acid oxidation was monitored spectrophotometrically at λmax 234 nm corresponding to maximum absorption of octadienoic acids hydroperoxides. The activity of the compound as a lipoxygenase inhibitor was characterized by the degree of inhibition I (%) of hydroperoxides accumulation after 5 min incubation with the 1mM of test compounds (Table 6). It was found that all compounds are mild inhibitors of LOX-1B with I ranged between 1-19%.
Influence of compounds on lipid peroxidation of rat liver homogenates and mitochondrial functions
In this study the influence of tested compounds on lipid peroxidation (LP) in rat liver homogenates induced by Fe3+ and tert-butylhydroperoxide (tBHP) was followed. The accumulation of products that reacted with thiobarbituric acid (TBARS) was monitored spectrophotometrically at λmax 530 nm.63 The IC50 values (the concentration of a compound required for achieving in vitro 50% inhibition of the reaction) are given in Table 7.
|
Compound |
IC50, μM |
|
|
Fe3+ |
tBHP |
|
|
1 |
22.8±6.4 |
11.1±3.9 |
|
2 |
14.0±2.8 |
5.3±1.0 |
|
3 |
24.1±6.4 |
3.7±1.1 |
|
4 |
3.4±0.8 |
0.9±0.2 |
|
5 |
3.4±0.7 |
0.5±0.1 |
|
6 |
3.9±1.0 |
0.8±0.2 |
|
1a |
11.4±2.0 |
10.9±1.8 |
|
2a |
6.0±2.6 |
26.7±7.1 |
|
3a |
6.2±2.2 |
19.8±6.2 |
|
4a |
0.9±0.6 |
2.4±0.5 |
|
5a |
4.0±3.2 |
1.2±0.2 |
|
6a |
4.9±1.3 |
1.7±0.4 |
|
BHT |
0.76±0.11 |
3.98±0.75 |
Table 7 Influence of compounds on tBHP and Fe3+ induced lipid peroxidation
Among tested compounds in tBHP induced LP the most effective antioxidants with IC50 values ranging between 0.5–0.9µM were compounds with –(CH2CH2)- spacer 4-6. However, their effect on Fe3+ induced lipid peroxidation is less pronounced. Antioxidant activity of 4-6 was 6 times higher than that of 1-3.
Mitochondria play the key role in O2-dependent energetic support of cells and accordingly in the production of ROS, participate in the apoptotic cascade by serving as a convergent center of apoptotic signals originated from both the extrinsic and intrinsic pathways. The change in mitochondrial potential is an indicator of both the activity of the respiratory chain of mitochondria and the induction of proapoptotic mitochondrial permeability transition pore opening.64 We examined the influence of compounds on mitochondrial potential in the presence of complex I and complex II substrates (Table 7) and on Ca-induced mitochondrial swelling as the direct indicator of mitochondrial permeability transition pores opening.
It was shown that all compounds in concentration up to 30 μM don’t affect the membrane potential ∆φ and Ca-induced swelling of isolated rat liver mitochondria that is an important feature of pharmaceutical agents. Further studies of the effect of the compounds (at 10µm) on the viability of rat brain cortical neurons in primary culture also did not reveal the significant cytotoxicity of these compounds (data not shown) and these results allow us to propose the future investigations of modified 2,6-di-tert-butylphenols with pyridine moiety as non-toxic antioxidant as cytoprotectors against oxidative stress.
The synthesis, structures, redox properties and in vitro antioxidant effects of new pyridine with 2,6-di-tert-butylphenols groups and their water-soluble hydrochlorides are presented. The obtained compounds are characterized by NMR, IR, X-ray and elemental analysis. Antioxidant activity is evaluated using model reactions of hydrogen atom abstraction (DPPH-test) and one-electron reduction (CUPRAC-test), cyclic voltammetry (CV) as well as by biochemical assays. It was shown that compounds possess radical scavenging activity of prolonged action due to the formation of relatively stable phenoxyl radicals as evidenced by EPR. The compounds are found to be mild inhibitors of lipoxygenase. Both lipophilic and hydrophilic forms demonstrate high antioxidant activity in induced lipid peroxidation of rat liver homogenates in vitro. Moreover, the introduction of the -CH2- spacer leads to a significant increase of antioxidant properties. It is also shown that compounds don't affect calcium-induced mitochondrial swelling and the mitochondrial membrane potential. No cytotoxicity was found for the compounds. Results of this study open the scopes for the search of novel water-soluble drugs with antioxidant properties.
The financial support of RFBR № 18-03-00203, 19-33-90236, 20-03-00471 (synthesis, electrochemical and EPR studies), and RSF № 19-13-00084 (experiments on lipid peroxidation) is gratefully acknowledged. Studies of mitochondrial functions and influence on cell viability were supported by Russian State assignment № 0090-2019-0005. The EPR measurements were performed using spectrometer of MSU Chemistry Department "Nanochemistry and Nanomaterials" Equipment Center, supported by Moscow State University Program of Development. The equipment of Center for Collective Use IPAC RAS (agreement N14.621.21.0008, ID RFMEFI62114X0008) was used in the biological experiments.
We confirm that there are no known conflicts of interest associated with this publication.

©2020 Nikitin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.