eISSN: 2379-6367


Research Article Volume 10 Issue 4
1Laboratory of Mycology and Parasitology, Institut Pasteur, Ivory Coast
2Immunology Laboratory, Pasteur Institute, Ivory Coast
3Laboratory of Chemical, Food and Environmental Process Sciences, INPHB, Ivory Coast
4Laboratory of Biotechnology and Valorization of Agroresources, Peleforo Gon Coulibaly University, Ivory Coast
5UFR Agriculture, Fishery resources and Agro-industries, University of San Pedro, Ivory Coast
Correspondence: Ousmane Coulibaly, Department of Agriculture, Fishery resources and Agro-industries, University of San Pedro, BP 1800 San-Pedro, Ivory Coast, Tel +2250707814424
Received: July 05, 2022 | Published: August 5, 2022
Citation: Coulibaly O, Soro PL, Kamagate T, et al. Antifungal activities of Erythrina senegalensis hydroalcoholic extract partitions on the resistant germs responsible for candidiasis in HIV-infected subjects. Pharm Pharmacol Int J. 2022;10(4):124-128. DOI: 10.15406/ppij.2022.10.00374
Since the mortality rate of candidiasis in HIV-infected subjects is very high, Erythrina senegalensis, an Ivorian medicinal plant, would be an alternative to the problem of patient care. It is therefore a question of evaluating in vitro the antifungal activity of Erythrina senegalensis leaves partitionned extracts on resistant strains of Candida albicans.
Methods of double dilution in liquid medium and diffusion in solid medium were used for the antifungal tests carried out on the partitions of Erythrina senegalensis leaves hydroalcoholic extract. Among partitions obtained, the hexane partition was the most active with a MIC of 3.12 mg/ml, an IC50 of 0.95 mg/ml on Candida albicans. With an inhibition diameter of 14 mm, this partition has antifungal activity on strains of Candida albicans resistant to amphotericin B and fluconazole, which are antifungals prescribed to PLHIV suffering from candidiasis.
The hexane partition of Erythrina senegalensis therefore has a fungistatic action on resistant strains of Candida albicans. This score could therefore be used as an Improved Traditional Medicine (ITM) in the treatment of fungal infections in HIV-infected subjects.
Keywords: Erythrina, hexane, HIV, candidiasis
Candidiasis is a yeast infection caused by Candida albicans, encapsulated yeast-like fungi that mainly infect people with a weakened immune system.1 Having become the main factor favoring these candidiases, HIV infection has modified the epidemiology of these opportunistic diseases. In sub-Saharan Africa, candidiasis, which remains the major cause of opportunistic mycoses, ranks fourth among deaths from infectious diseases.2,3 This very high mortality rate among subjects infected with HIV in our developing countries would be due to the inaccessibility of conventional antifungals to our populations given their very high cost. Also, some studies have shown the renal and hematological toxicity of Amphotericin B.4 Thus, to solve this problem of the management of candidiasis in HIV-infected subjects, the alternative search for new, more effective and less expensive molecules in our floristic heritage is necessary. Hence this interest in Erythrina senegalensis, a plant used in traditional Ivorian medicine against candidiasis. Called respectively "Timini" and "Kindjê" by Malinké and Akan ethnic groups of Côte d'Ivoire, Erythrina senegalensis is a thorny shrub 6 to 7 m high which is widespread in West Africa.5 Leaf extracts are indicated in the treatment of oral diseases, dermatoses, abdominal pain, bronchial infections and cough.6
This study, which is part of the search for a more effective and suitable antifungal phytomolecule, is a contribution to the fight against candidiasis in HIV-infected subjects. It will consist in determining the antifungal activity of partitions resulting from the total hydroalcoholic extract of Erythrina senegalensis leaves, on Candida albicans in comparison with classic antifungals regularly prescribed to patients infected with HIV. It will also be a question of carrying out a phytochemical screening in order to identify chemical groups responsible for these so-called antifungal activities.
Material
Plant material
The plant material consists of of Erythrina senegalensis leaves (Fabaceae) located in the south-west of Côte d'Ivoire whose geographical coordinates are: 5°47′08″ North - 6°36′29″ West, Altitude: 134 meters. This plant has been authenticated at the National Floristic Center of Félix Houphouët Boigny University of Cocody in Côte d'Ivoire (Figure 1).
Biological material
The fungal pathogen used in this study is the susceptible Candida albicans isolate MY18-22998/18346 and the Candida albicans isolate MY18-53502/18870 resistant to Amphotericin B and Fluconazole. These different strains were isolated from samples taken from patients infected with HIV/AIDS in the Institut Pasteur mycology laboratory in Côte d'Ivoire. They were cultured in a culture medium consisting of Sabouraud chloramphenicol agar (Bio Mérieux).
The reference commercial antifungals that we used are: Amphotericin B (AB100 Lot 5B 3026 Bio-Rad); 5-Fluorocytosine (5FC1 Lot 4L 3023 Bio-Rad); Fluconazole (FCA25 Lot 5C 3028 Bio-Rad).
Methods
Plant collection
The leaves of Erythrina senegalensis were harvested, washed and then dried in the shade. After three weeks of drying, the leaves, which had become dry, were ground in an IKA-MAG grinder. The fine powder obtained after grinding was stored in glass jars in the freezer at -4°C.
Preparation of the hydroalcoholic total extract7
One hundred (100) grams of Erythrina senegalensis powder were dissolved in 500 ml of a 70% ethanol solution and then homogenized for 24 hours at ambient room temperature, using the IKA-MAG magnetic stirrer. After decantation, the supernatant was collected, filtered twice on absorbent cotton then on Wathman paper 3 mm in diameter. The filtrate obtained was evaporated using a BUCHI rotary evaporator. The resulting paste was dried in an oven at 60°C until a dry powder was obtained which constituted the total hydro-alcoholic extract.
Partitioning of the total hydroalcoholic extract8,9
The hydroalcoholic extract obtained was introduced into a separatory funnel and exhausted three times with 150 mL of hexane. After decantation, the hexane phase was recovered, dried over magnesium sulphate and then filtered using 3 mm diameter WATTMAN paper. The hexane was removed on the BUCHI rotary evaporator and the resulting hexane partition powder (Fhex) was stored in a dark jar in the EXPRESSCOOL refrigerator at -4°C until use. The same operation was successively carried out with dichloromethane (Fdcm) and ethyl acetate (Face). At the end, the aqueous partition was evaporated and then dried in a MEMMERT type oven at 60°C.
Yield calculation
The yield of the partitions of Erythrina senegalensis leaves ETHA extract was calculated according to the following formula:
Rd = (m X 100) / M
Rd: partitioning efficiency (%);
m: mass of the partition (g);
M: mass of the plant extract (g)
Preparation of the inoculum
Using a platinum loop, a young colony of Candida albicans was taken and homogenized in 10ml of sterile distilled water to obtain the stock suspension (100) concentrated at 106 cells/ml. From the suspension (100), a second suspension (10-1) is prepared by dilution to 1/10th of the first in order to obtain a suspension of 105cells/ml which constituted the fungal inoculum.
Carrying out evaluation tests
The evaluation tests make it possible to determine the antifungal parameters (MIC, IC50, MFC) thanks to the method of double dilution in an inclined tube.7,10
For each of the partitions of the ETHA extract from the leaves of Erythrina senegalensis, a series of 12 test tubes was prepared. It included 10 test tubes and 2 control tubes, one of which without plant partition constituting the control of growth of the fungal pathogen and the other without plant partition and without fungal pathogen serving as control of sterility of the agar medium. Thus, 2g of plant partition were homogenized in 20ml of Sabouraud chloramphenicol agar liquid at 40°C in tube T1 in order to obtain a concentration of 100mg/ml in this tube. Half of this T1 tube was transferred to a T2 tube containing 10 ml of agar in order to obtain a concentration of 50 mg/ml in this T2 tube. This operation is repeated successively for the other tubes until tube T10 which obtained the lowest concentration of 0.195 mg/ml. The 10 test tubes therefore made it possible to obtain a range of decreasing concentrations of plant partitions ranging from 100 mg/ml to 0.195 mg/ml according to a geometric relationship of ratio 1/2. After sterilization in an autoclave at 121°C for 15 min, the test tubes obtained were tilted at room temperature to allow the cooling and solidification of the agar containing the partitions of Erythrina senegalensis leaves ETHA extract.
The prepared fungal inoculum (105 cells/ml) is used to inoculate the 12 prepared test tubes except the sterility control tube. The test tubes were then incubated at 37°C. for 48 hours and then the colonies present in each tube were counted by direct counting (the tests were repeated 3 times). The growth of the germ in each test tube is expressed as a percentage of survival (S) and calculated according to the following formula:
S= n / N x 100
S=% survival
n=number of colonies in the test tube
N=number of colonies in the control tube
The processing of these experimental data makes it possible to determine the antifungal parameters which are the MIC, IC50 and MFC:
Carrying out efficiency tests
The antifungal activity of the extracts was confirmed by the method of diffusion in agar medium using discs soaked in plant partitions or reference antifungals described by Traore et al.9 The principle of this method is based on the diffusion from a disc, of the active antifungal substance in the agar containing the fungal germ with the creation of a concentration gradient.
Sterilized disks 6mm in diameter cut out of Wathman paper are placed in the partitions of Erythrina senegalensis leaves ETHA extract for 1 hour. The prepared fungal inoculum (105 cells/ml) is spread on the surface of the Sabouraud-chloramphenicol agar poured into a sterilized Petri dish and then dried at 37°C for 5 min. In each inoculated Petri dish, the discs thus soaked with plant partitions are placed next to discs soaked in Amphotericin B and Fluconazole, which are left to incubate at 37°C for 48 hours. Around each disc containing a partition or a reference antifungal, inhibition diameters are observed and measured (the test was repeated 3 times for each partition).
Phytochemical screening
Phytochemical screening was carried out with the aim of detecting the major chemical groups contained in the total extracts of Erythrina Senegalensis leaves. All these coloring and precipitation reactions are summarized in Table 1.8,11-15
Chemical groups |
Reagents |
Results |
Sterols and terpenes |
Acetic anhydride |
Crimson or violet ring |
Alkaloids |
Sulfuric acid |
White precipitate |
Polyphenols |
2% hydrochloric acid solution Mayer's reagent |
Blue-blackish coloring |
Flavonoids |
1% alcohol solution of ferric chloride |
Red or orange coloring |
(Flavones) |
Concentrated hydrochloric acid |
Purplish cherry-red or reddish-brown coloration |
Anthocyanins |
Magnesium shavings |
Red precipitate soluble in amyl acid |
Catechic tannins |
hydrochloric alcohol |
Blue-black coloring |
Gallic tannins |
Isoamyl alcohol |
Yellow or red coloring |
Table 1 Chemical group identification tests
Yield of partitions from Erythrina senegalensis leaves hydroalcoholic extract
The partitionings carried out on the hydroalcoholic extracts of Erythrina senegalensis leaves made it possible to obtain the partitions with hexane (Fhex), with dichloromethane (Fdcm), with ethyl acetate (Face) and final aqueous (Faqf) with yields recorded in Table 2.
|
Plant partition |
Yields (%) |
|||
|
Fhex |
Fdcm |
Face |
Faqf |
|
|
Erythrina senegalensis |
45,83±0,36 |
14,56±0,72 |
8,28±1,16 |
31,33±2,05 |
Table 2 Yields of Erythrina senegalensis partitions
Fhex, hexane partition; Fdcm, dichloromethane partition ; Face, ethyl acetate partition; Faqf, final aqueous partition
The yield of the Fhex partition of Erythrina senegalensis ETHA extract (45.83%) is higher than those of Fdcm, Face and Faqf partitions which are respectively 14.66%; 8.28% and 31.33%.
Antifungal activity of partitions from Erythrina senegalensis leaves hydroalcoholic extract
After 48 hours of incubation at 37°C, the growth of Candida albicans colonies in the test tubes, expressed as a percentage of survival (S), is is translated by sensitivity curves presented in Figure 2.
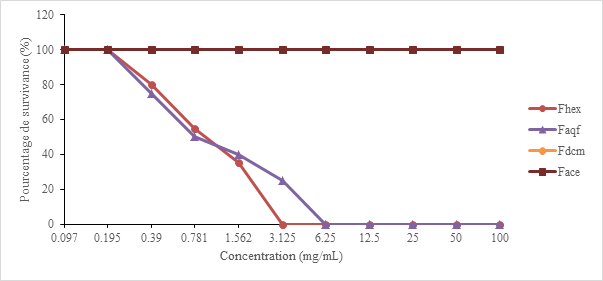
Figure 2 Sensitivity curves of the partitions from Erythrina senegalensis leaves hydroalcoholic extract on Candida albicans.
The MIC of the Fhex and Faqf partitions of Erythrina senegalensis are respectively 3.12 mg/mL and 6.25 mg/mL on Candida albicans while the Fdcm and Face partitions of the same plant show no antifungal activity on Candida albicans which maintains 100% survival. CMF was researched experimentally and none of the new concentrations applied could prevent the reappearance of colonies after 48 hours of reincubation. The Fhex and Faqf partitions of Erythrina senegalensis therefore have a fungistatic effect on Candida albicans strains.
Effectiveness of Erythrina senegalensis active partitions on sensitive and resistant strains of Candida albicans
The evaluation of the most active partition (Fhex) resulting from the hydroalcoholic extract of Erythrina senegalensis leaves made it possible to obtain in Petri dishes, diameters of growth inhibition of Candida albicans strains sensitive and resistant expressed by the histograms of Figures 3 & 4.
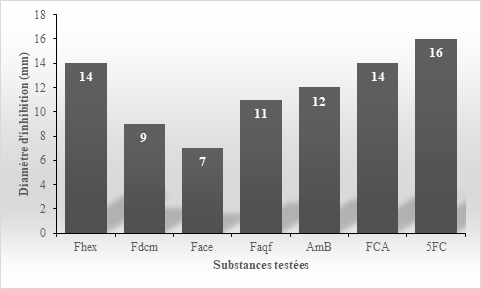
Figure 3 Efficacy of partitions from Erythrina senegalensis ETHA extract and reference antifungals on Candida albicans susceptible strains.
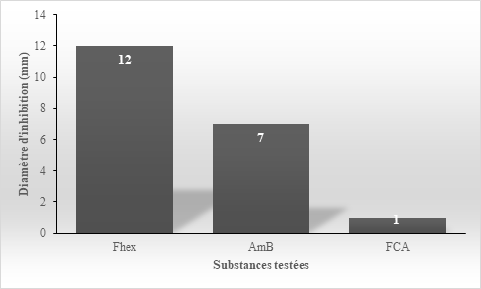
Figure 4 Efficacy of Erythrina senegalensis hexane partition (Fhex) and reference antifungals on Candida albicans resistant strain.
The inhibition diameter of Fhex, Faqf partitions and AmB, FCA and 5FC antifungals which are 14mm, 11mm, 12mm, 14mm and 16mm respectively, are above the sensitivity threshold of 10mm unlike Fdcm partitions and Face which have respective inhibition diameters of 9mm and 7mm.
The inhibition diameter of Erythrina senegalensis leaves Fhex partition (12mm), on Candida albicans resistant strain is greater than those of Amphotericin B (7mm) and Fluconazole (1mm), which are lower at the sensitivity threshold of 10mm.
Phytocompounds of the partitions of Erythrina senegalensis hydroalcoholic extract
Phytochemical screening made it possible to detect in the partitions of Erythrina senegalensis hydroalcoholic extract, the different families of secondary metabolites present. The results obtained are shown in Table 3.
Families of secondary metabolites |
Partitions |
|||
Fhex |
Fdcm |
Face |
Faqf |
|
Sterols and Terpenes |
+ |
- |
- |
+ |
Alkaloids |
- |
- |
- |
- |
Polyphenols |
+ |
+ |
- |
- |
Flavonoids |
+ |
- |
- |
+ |
Anthocyanins |
- |
- |
- |
- |
Tanins |
- |
- |
- |
- |
Quinones |
- |
- |
- |
- |
Table 3 Families of secondary metabolites contained in the partitions from Erythrina senegalensis leaves hydroalcoholic extracts
(+), presence ; (-), absence ; Fhex, partition with hexane ; Fdcm, partition with dichloromethane ; Face, partition with ethyl acetate ; Faqf, final aqueous partition
The Erythrina senegalensis partitions richest in families of chemical groups are the Fhex and Faqf partitions which have in common sterols and terpenes, as well as flavonoids. The Fhex partition additionally contains polyphenols. The Fdcm and Face partitions are the poorest in secondary metabolite families. Also none of the partitions contain alkaloids, tannins, quinones and anthocyanins as was the case for the extract.
The objective of this work is to search in extracts of Erythrina senegalensis leaves, for antifungal substances with the same effectiveness as the reference antifungals against germs responsible for opportunistic candidiasis in HIV/AIDS.
The successive partitioning of Erythrina senegalensis leaves hydroalcoholic extract with solvents of increasing polarity made it possible to obtain partitions whose yields vary from 8.28% to 54.31%. The hexane partition gave the best yield because according to Golly et al.,16 hexane is generally used for defatting plant powder extracts. Thus, a high yield of hexane partitioning could be due to a high presence of lipid compounds in the leaves of Erythrina senegalensis.
Among the partitions from Erythrina senegalensis hydroalcoholic extract, only the Fhex and Faqf partitions resulted in a clear and effective inhibition of Candida albicans with respective MICs of 3.125 and 6.25mg/mL. As for the Fdcm and Face partitions, no significant antifungal activity was observed in the test tubes. These answers are justified by the principle of successive partitioning which consisted in using several solvents of different polarities in a precise order to extract all the extractable compounds solvent after solvent. The order of increasing polarity of the solvents being hexane, dichloromethane, ethyl acetate and water; the bioactive compounds are isolated and concentrated progressively according to their polarity, during the partitioning.8,9 Thus the fungistatic activity of the Fhex partition of Erythrina senegalensis would be due to the content of this plant in lipid compounds, in particular sterols and polyterpenes.16
The low antifungal activity of the Faqf partition, could be explained by the fact that, being the last polar solvent used, water recovered very few bioactive compounds that had already been extracted by hexane as previous solvents.
Efficacy tests carried out on sensitive and resistant strains of Candida albicans have made it possible to compare the antifungal activity of the hexane partition of Erythrina senegalensis leaves with that of Amphotericin B and Fluconazole, antifungals regularly used for the treatment of candidiasis in subjects infected with HIV.
With the sensitive strain of Candida albicans, the inhibition diameters of the Fhex, Faqf and antifungal partitions tested are above the sensitivity threshold of 10mm. This means that the Fhex partition is as effective as Amphotericin B, Fluconazole and 5-Fluorocytosine on the susceptible strain of Candida albicans. Indeed, according to Biyiti et al.,17 a substance is said to be active on a germ when its inhibition diameter is greater or equal to 10mm.
However, on the resistant strain of Candida albicans, the inhibition diameters obtained with Amphotericin B and Fluconazole are below the sensitivity threshold of 10mm, unlike the hexane partition. This confirms the ineffectiveness of these reference antifungals and the effectiveness of this Fhex partition of Erythrina senegalensis on the strain of Candida albicans resistant to Amphotericin B and Fluconazole. According to Ziouti et al.,18 resistance and sensitivity are not always linked to the presence of a single compound or a single class of molecules; these are complex mechanisms that involve several types of molecules. This could therefore explain the effectiveness of the Fhex partition of Eryhtrina senegalensis on the resistant strain of Candida albicans.
The phytochemical screening revealed in the Fhex partition which is the most active of the hydroalcoholic extract of Erythrina senegalensis leaves, the common presence of sterols and terpenes and flavonoids. These results are in agreement with the work of Ligor et al.,19 for whom hexane is one of the most widely used solvents for the extraction of polyphenols (flavonoids.) and polyterpenes (sterols and terpenes). According to Owoseni et al.20, these families of secondary metabolites are well known for their antimicrobial activities. Indeed, thanks to their free hydroxyl groups, flavonoids and sterols and terpenes of Erythrina senegalensis Fhex partition could lead to the death of fungal pathogens by the destruction of cell membranes following a total loss of homeostasis.21,22
At the end of this study, we can affirm that the partitioning of Erythrina senegalensis hydroalcoholic extract made it possible to obtain the partition with hexane which has a fungistatic activity on the sensitive and resistant strains of Candida albicans more interesting than that of amphotericin B and fluconazole which are the antifungals commonly prescribed to AIDS patients suffering from cryptococcosis. These classic antifungals having shown their limits in the treatment of candidiasis, the use of this medicinal plant as a more effective and less expensive improved traditional medicine (MTA) could be beneficial for the treatment of candidiasis in HIV-infected subjects. In addition, more in-depth studies by chromatography and spectroscopy will make it possible to purify the active principle of the extracts of Erythrina senegalensis in order to give better results and to know its chemical nature.
None.
Authors declare that there is no conflict of interest.

©2022 Coulibaly, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.