eISSN: 2379-6367


Review Article Volume 8 Issue 1
1Universite de Kolwezi, RD Congo
2CEBCE, Kivu, RD Congo
3University of Mbarara, Uganda
4IFBV-BELHERB, Luxembourg
Correspondence: Pierre Lutgen, IFBV-BELHERB, L-6988, Luxembourg
Received: January 02, 2020 | Published: February 13, 2020
Citation: Munyangi J, Gisenya P, Ogwang P, et al. An unexpected, revolutionary property of Artemisia infusions: immunoglobulins in the skin lead to a long-lasting prophylaxis. Pharm Pharmacol Int J. 2020;8(1):46-62. DOI: 10.15406/ppij.2020.08.00280
Naturally acquired immunity to malaria is a known phenomenon. But little is known about the underlying mechanisms. The majority of adults in sub-Saharan Africa rarely experience overt disease, despite they have a population of parasites in their blood that could prove lethal to a malaria-naïve visitor. The use of Artemisia herbal medicine is spreading in many African countries, in schools, health centers. It was shown 10 years ago in Uganda that regular drinking of infusions from Artemisia plants had a strong prophylactic effect. We were of the belief that this prophylaxis was going to last for a few days, maybe a few weeks. Over the last 2 months we received several surprising inputs indicating that this prophylaxis was lasting for months, even years. But more recently several partners also report that when Artemisia infusions or capsules have been used during 7 days to cure a malaria infection, the people having used this short-term treatment also experience a long-lasting prophylaxis. The most interesting research lead is that specific IgEs induced by Artemisia consumption remain for months in the skin. In this review paper we present several hypotheses to explain this revolutionary property of Artemisia plants, and more particularly Artemisia afra used since generations in many African countries against tropical diseases.
Keywords: artemisia, plasmodium, immunoglobulins, gametocytes, prophylaxis
The prophylactic efficiency of Artemisia annua was first discovered and documented by Patrick Ogwang from Uganda in several scientific papers.1 Patrick Ogwang also developed ARTAVOL® in collaboration with scientists at Makerere University. Artavol is a beverage for malaria prevention at household level: a blend of Artemisia annua (without artemisinin)-avocado powder (Persea americana)-lemon grass (Cymbopogon citratus). In the research conducted by the team, it was found that after eight months of taking the beverage, the subjects did not develop any fevers associated with malaria. Among the study participants, hospital visits for fever related causes fell by 80 per cent as compared to the control subjects. In 2014 the Ministry of Health in Uganda recognized the innovation and awarded the principal investigator for outstanding achievement in research for herbal prevention of malaria. Artavol production currently stands at 6,000 one hundred-gram tins per month.2
Initially, we were of the belief that regular drinking of Artemisia infusion, 3-5 cups per week, had a prophylactic effect which lasted for a few days, maybe a few weeks. But over the last 2 months we received several surprising inputs indicating that this prophylaxis was lasting for months, even years. First a recent personal communication from Patrick Ogwang: 10 volunteers who had been free of malaria for years whilst regularly drinking Artemisia annua tea decided to stop drinking tea and in the two following years they remained free of new malaria infections. A similar observation was made by Dr Jérôme Munyangi. 200 primary school children, who had been drinking Artemisia tea in 2015 in a large-scale clinical trial failed to continue, because not enough Artemisia was available. 3 years later, prophylaxis does still work and they are free of malaria, as certified by the medical director of the local hospital in Maniema-RDC.
During our meeting on November 20, 2019 with the 5 partners of the project “Save and healthy children in Togo and Benin” similar long-lasting effects were described by several partners, concerning not only children but also adults. The adults had been cured from a malaria infection by drinking the mandatory 21 cups over 7 days and since then had no reinfection. The children in these institutions are free of malaria since years. This protection against reinfection observed after an Artemisia infusion treatment was never reported after an ACT treatment or clinical trials. To the contrary, in a large trial in Liberia, a reinfection rate 61% for ASAQ and 40% for AL was noticed on day 42.3 Independently of the first reports of Ogwang and Munyangi we received on November 31, 2019 a similar report from Soeur Sylvie at Goma (RDC). She is Mother Superior of the community of Ursuline sisters and headmistress of a secondary school at Goma. She has developed Artemisia annua and Artemisia afra plantations with the help and guidance of Dr Pascal Gisenya. Sister Sylvie reports that 80 children, who had been cured from a malaria infection by the usual 7 days treatment, have not suffered from another attack since that date. This also applies to the farmers in the plantations who stayed malaria free for the recent 6 months.
Dr NB Daddy, director of a large hospital in Goma (RDC), reports a similar story. He is the medical doctor, who had run with P Weathers a clinical trial documenting that Artemisia infusions stayed efficient and saved the life of 18 patients where ACT pills had completely failed. He now reports that 24 patients who had received this Artemisia treatment 12 months ago never had a relapse or reinfection.4 In 2014 we had already received a similar observation from Dr Felicitas Roelofsen in India. When people take the Artemisia tea for 7 days they do not come back with a malaria infection during a full year. If they take the 3 day Coartem cure, they come back several times per year for treatment. Studies of malaria immunity conducted in the “pre-internet” and “pre-PubMed” eras illustrate how science advances in an unpredictable yet steady progression of insights that may not be appreciated when first reported. In 1937, it was reported that passive transfer of sera from chronically infected rhesus monkeys to malaria naïve monkeys challenged with Plasmodium knowlesi infected red blood cells protected the recipients from high-density parasitemia and death.5,6
Human antibody responses to mosquito salivary components also could represent a promising tool for evaluating the human-vector contact. The saliva of the mosquito bite contains molecules which promote the invasion of sporozoites into the blood stream, but others create antibodies which after repeated bites prevent this invasion and are prophylactic. Mosquito saliva also contains components to facilitate blood feeding, which could have an impact on infection. A protein was found, which negatively influences sporozoite movement in the host.7 Naturally acquired immunity to falciparum malaria protects millions of people routinely exposed to Plasmodium falciparum infection from severe disease and death. There is no clear concept about how this protection works; much less is there a consensus regarding the mechanism(s) of protection. It may virtually be 100% effective against severe disease and death among heavily exposed adults. The induction of an adult-like immune status among high-risk infants in sub-Saharan Africa would greatly diminish disease and death. It would be more efficient than the commercial vaccines, which presently swamp Africa in clinical trials, with disastrous results and side effects.
Immunoglobulins in malaria endemic areas
IgE is an immunoglobulin isotope which can only be found in mammals. In the average population its concentration is very low at 10 to 300 ng/ml.8 It is linked to and activated by two receptors: with a high affinity for Fcε RI and a low affinity for Fcε RII or CD23.9 The crosslinking of CD23 with macrophages or other effector cells carrying CD23 complexes containing IgE might play an important role in the immunity against malaria involving TNF mechanisms.10 The total IgE level in a population is strongly related to the malaria endemicity in that area and are routinely over 1000 ng/ml with many values over 2000 ng/ml.11
|
Country |
IgE ng/ml |
|
Sweden |
8 |
|
Madagascar |
301 |
|
Thailand |
647 |
|
Liberia |
2134 |
Specific IgE and IgG4 antibody (Ab) responses to Aedes aegypti saliva were evaluated in young Senegalese children living in an area of exposure to the Aedes vector. Specific IgE and IgG4 responses increased during rainy season of high exposure to Aedes bites.12
This was also studied in Thailand. Forty adult Thai patients with acute falciparum malaria who had subsequent recrudescent infections and 40 patients matched for age, therapeutic regimen, and disease severity who were cured by Day 28 were studied. All cured patients had positive immunoglobulin (Ig) G in their admission plasma, compared with only 60% of patients who failed to respond to treatment The proportion of IgM-positive cases at admission was also higher in the successfully treated group than in the group with failure (70% versus 30%). The patients with uncomplicated malaria who were both IgG and IgM positive at admission had significantly shorter fever clearance times and lower admission parasitemia levels compared with those who were negative. These results suggest that antimalarial antibodies may play an important supportive role in the therapeutic response to antimalarial drugs during acute falciparum malaria.13,14
IgE in association with monocytes or platelets may give rise to reactions that are protective and/or pathogenic. Most children and adults living in areas where the endemicity of Plasmodium falciparum malaria is high, have significantly elevated levels of both total IgE antibodies and specific antimalarial IgE bodies in blood. IgE containing immune complexes are known to give rise to monocyte activation via the NO transduction pathway. A recent study in Nigeria shows that the malaria infection specifically raises IgE, but that IgG and IgM remain virtually stable. There is a strong positive correlation between IgE and parasite density. IgE raises almost exponentially with the severity of the disease. The increase is the same for males and females as has been shown in several studies (Table 1 & 2).15-22
Immunoglobulin levels |
Male control subjects |
Male infected subjects |
P-value |
IgG (g/dl) |
14.59± 0.57 |
13.29±0.78 |
P>0.05 |
IgM (g/d1) |
1.77±0.08 |
2.23±0.58 |
P>0.05 |
IgE (IU/ml) |
35.39±36.19 |
613.03±295.22 |
P<0.05 |
Table 1 Immunoglobulin levels in malaria infected and non-infected males
Immunoglobulin levels |
Female control subjects |
Female infected subjects |
P value |
IgG (g/dl) |
15.32±0.48 |
13.25±0.92 |
P>0.05 |
IgM (g/dl) |
1.67±0.10 |
2.35±0.64 |
P>0.05 |
IgE (IU/ml) |
40.05±40.87 |
639.24±315.44 |
P<0.05 |
Table 2 Immunoglobulin levels in malaria infected and non- infected females
In a trial in Thailand on 89 malaria infected patients compared with healthy patients from a non- endemic area the average IgE level was 3 700 vs 114.23 High levels of IgE are also detected in asymptomatic carriers.24 The elevation of specific Plasmodium falciparum antibodies is age dependent. The prolonged and repeated exposure to malaria parasites is necessary for the induction of these specific antibodies and there is a significant correlation between their level and the number of malaria attacks.25 The ability to resist Plasmodium falciparum malaria is an important adaptive trait of human populations living in endemic areas. The detection of significant differences in the expression of this trait and the identification of the factors involved should improve the understanding of the host-parasite relationship and might lead to advances in control strategies. In a study in Tanzania it was clearly demonstrated that high anti-Plasmodium falciparum IgE levels were associated with reduced acute risk of acute malaria in all age groups, independently of the total IgE level. High levels of IgG weren’t associated either with a reduced risk to succumb to a new clinical episode.26,27
A study in Senegal relates the clinical protection to IgG. This study evaluated antibody dependent respiratory burst activity (ROS) in neutrophils induced by Plasmodium falciparum merozoites and antibodies in the sera of two different African endemic populations and investigated its association with naturally acquired clinical protection. 230 sera were analyzed from individuals of all age groups living in meso-(Ndiop) or holo-endemic (Dielmo) Senegalese villages. Strikingly, individuals in Dielmo were seventeen-fold less susceptible to malaria attacks. ROS activity correlated with merozoite IgG1 and IgG3 antibody titers.28 Research has shown that, for killing, the antibody molecule activates additional systems. The immune system seems to have a previously unrecognized chemical potential intrinsic to the antibody molecule itself. All antibodies studied, regardless of source or antigenic specificity, can convert molecular oxygen into hydrogen peroxide (H2O2), thereby potentially aligning recognition and killing within the same molecule. Antibodies can generate hydrogen peroxide (H2O2). This process is catalytic. Antibodies produce up to 500 mole equivalents of H₂O₂.29-32
Reactive oxygen species (ROS) release by activated neutrophils might be more involved in immune protection from malaria than generally appreciated. Neutrophils are abundant in the circulation and are one of the immune system's first lines of defense against infection. There has been substantial work carried out investigating the role of neutrophils in malaria and it is clear that during infection neutrophils are activated and are capable of clearing malaria parasites by a number of mechanisms.33,34 Breast milk protects neonates against malaria and other diseases during six months. Besides lactoferrin, breastmilk is also very rich in hydrogen peroxide. Colostrum contains up to 24 000 microM/L, thousand times more than in human blood!35 IgE leads to the production of histamine via the so-called histamine release factor (HRF). This histamine release via IgE was also noticed in AIDS patients. IgG does not generate this release.36 The effect of histamine in malaria has been studied already 50 years ago. Extracts of the blood of monkeys (Macaca mulatta) infected with Plasmodium knowlesi contain histamine. No histamine was detected in the blood of healthy monkeys. This may explain to a large extent the lasting immunological memory to blood stage infection.37,38
Specific immunoglobulins against sporozoites and merozoites
The seminal work of Druilhe and Mazier has shown in 1986 that there are sporozoite specific antibodies alongside the merozoite specific ones. In two African villages which differed in the level at which mosquitoes transmit the disease, both the prevalence by age group and the levels of anti-sporozoite antibodies differed markedly. In the low-transmission area, these antibodies were not detected in subjects aged 2 to 10 years; thereafter, prevalence increased gradually with the age of the subject and reached 90% in subjects aged 50 to 80 years. In the high-transmission area, all of the subjects studied, including the younger ones, were positive. Anti-sporozoite antibody levels were independent of the levels of antibodies directed against blood stages. These results suggest that stage-specific antibodies reflect the cumulative number of sporozoites inoculated in humans by mosquitoes and may therefore have useful epidemiological applications. In addition, the presence of stage-specific antibodies in the sera of African adults collected at different times after departure from the endemic area indicates that they may last for several years. Seasonal variations of anti-sporozoite antibodies were estimated by titrating serum samples collected in May and in December from the same individuals. No seasonal difference in either prevalence or titer was noted. This result confirms the long remanence of those antibodies.
Subjects recovering from a first attack were found to be negative. Of 21 sera from seven European subjects who experienced a Plasmodium falciparum primary attack after a short visit to an endemic area, all were negative for sporozoite antibodies. Each of these individuals developed high titers of antibodies to blood stages.39 Other authors also describe these antibodies specific against sporozoites and different from those against blood stage parasites.40-42
Also long-lived IgM have been described. Most studies on human immunity to malaria have focused on the roles of immunoglobulin G (IgG). Analyzing multiple human cohorts to assess the dynamics of malaria-specific IgM during experimentally induced and naturally acquired malaria, in a recent study identified IgM activity against blood-stage parasites and found that merozoite-specific IgM appear rapidly in Plasmodium falciparum infection and is prominent during malaria in children and adults with lifetime exposure, together with IgG. Unexpectedly, IgM persisted for extended periods of time; no difference was found in decay of merozoite-specific IgM over time compared to that of IgG. IgM blocked merozoite invasion of red blood cells in a complement-dependent manner. IgM was also associated with significantly reduced risk of clinical malaria in a longitudinal cohort of children. These findings suggest that merozoite-specific IgM is an important functional and long-lived antibody response targeting blood-stage malaria parasites that contributes to malaria immunity.43
Serum samples from 158 West Africans were tested for antibodies against sporozoites. Antibodies specific for Plasmodium falciparum sporozoites were detected by means of the circumsporozoite precipitation assay and indirect immunofluorescence. More than 90 percent of the serum samples from adults gave positive immunofluorescent reactions against falciparum sporozoites, whereas most of the samples from children gave low or negative reactions.44 The detection of specific IgE antibodies is not always easy. A study from Brazil was able to improve the detection of specific IgE antibodies to Schistosoma mansoni and Leishmania chagasi by depleting IgG with protein G.45
The skin stage of malaria infection
Until recently, our knowledge of the molecular interactions between host and parasite during the early stage of malaria infection was limited. The number of sporozoites injected into the skin and penetrating the liver of the mammalian host is indeed very small. A review of the literature between 1970 to 1995 indicates that malariologists labored under the assumption that sporozoites rapidly left the inoculation site and significant interactions between host and parasite did not begin until sporozoites invaded hepatocytes and began to develop. Recent studies have now shown that sporozoites spend several hours at the inoculation site and initiate an immune response in the lymph nodes which drain this site, thus bringing this early stage of infection into the limelight. Exit from the inoculation site resembles a slow trickle and occurs over several hours. Sporozoites spend the majority of their extracellular time at the inoculation site, raising the hypothesis that this is where the malarial parasite is most vulnerable to antibody-mediated destruction. Some sporozoites do not enter the bloodstream and instead enter the lymphatic circulation and go to the draining lymph node. Studies have shown that approximately 15–20% of the inoculum ends up in the draining lymph node. These sporozoites, though at least initially alive, ultimately do not continue further and likely become fodder for the immune response. Several studies suggest that less than 20% of sporozoites successfully exit the dermis and travel to the liver (Figure 1).46-48
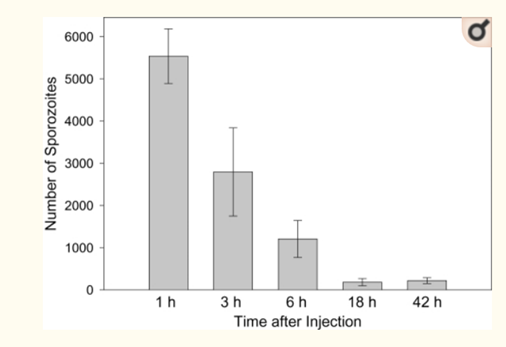
Figure 1 Kinetics with which sporozoites disappear from the inoculation site. Mice were injected intradermally in the ear with 5000 P. yoelii sporozoites, the ears were removed at the indicated time points and the number of sporozoites in each ear was quantified by PCR. There were four mice per group and the means+standard deviations are shown.
Antibodies could block infection in several ways, either by neutralizing sporozoites directly, opsonizing sporozoites for phagocytosis or blocking invasion of sporozoites into hepatocytes.49 Antibodies fix and activate human complement against Plasmodium falciparum sporozoites, which lead to sporozoite death.50 A recent paper highlights a very important finding. After plasma infusion, IgE in the vascular circulation and serum had a half-life of 2 days but positive skin tests results were still demonstrated after 50 days. This long-life of the IgE in the skin may play an important role in prophylaxis.51
Epithelia constitute an important barrier against invading microorganisms. Epithelial cells produce antimicrobial peptides and polypeptides, important effector molecules in the innate immune response in all species from insects to man. Cytokines also play a role in immunity of the skin to aggressions. Interleukin (IL)-1 for example is a highly active pro-inflammatory cytokine. Epidermal keratinocytes can secrete large amounts of IL-1α, which induces an inflammatory response in the skin. These epithelial cells might be critically involved in the immunity of the skin. This supports the concept that keratinocytes are important immuno-competent cells under physiological and pathological conditions. They are the most abundant cells in the epidermis.52,53 Cytokines are a double sword in the skin. Upon activation, monocytes and keratinocytes function to help reduce parasite burden through phagocytosis, cytokine production, and antigen presentation. However, they have also been implicated in in systemic inflammation and vascular dysfunction.54 In mycobacterial contaminations the infectant even tries to eliminate the keratinocytes.55
Keratinocytes also block other infections like leishmaniasis or schistosomiasis. IgE antibodies bind strongly to promastigotes.56-58 Immunoglobulins are associated with protection against malaria inoculation, by activating monocytes. The role of monocytes in malaria prophylaxis was first proposed by a research team from Uganda. Monocytes have a limited life span. In the absence of appropriate stimuli, they undergo apoptosis, but under the influence of survival signals, these cells differentiate into macrophages or dendritic cells. It has been shown that ligation of IgE on human monocytes markedly reduces the apoptosis. A cooperative, synergistic effect between immunoglobulins and monocytes was demonstrated. The addition of monocytes from healthy individuals to Plasmodium falciparum cultures in the presence of serum from immune individuals markedly inhibits the proliferation of the parasite in vitro. The activity of monocytes alone and immunoglobulins alone was moderate and inconsistent.59-61
Lactoferrin contained in milk selectively segregates into the skin and significantly reduces the sebum content and increases the number of keratinocytes, their migration and reduces their apoptosis.62-64 Human saliva also is rich in lactoferrin.65 Malaria-specific CD8⁺ T cells are primed in the skin draining lymph. Vertebrates maintain T cells for the detection of pathogens. Recognition triggers infection control. There are twice the number of T cells in the skin than in the peripheral blood. The efficiency achieved by this immunosurveillance depends upon the CD4 and CD8 T cell population. A significant reduction in anti-sporozoite CD8⁺ T cell response was observed in animals that had their draining lymph nodes removed prior to sporozoite infection.66 Immunity to malaria is stage-specific. CD4+ and CD8+ T cells inhibit early malaria infection stages, but not blood stage.67 Host cell traversal is another way for the sporozoite to find protection against the immune system. Host cell traversal protects the vulnerable sporozoite from phagocytosis, primes the sporozoite through the activation of apical exocytosis, and prepares the motile sporozoite for invasion.68
The neutralizing capacity of circulating antibodies is greater at the inoculation site than in the blood circulation. Furthermore, these antibodies are working, at least in part, by impacting sporozoite motility at the inoculation site. Using actively and passively immunized mice, it was found that most parasites are either immobilized at the site of injection or display reduced motility, particularly in their net displacement. And that antibodies severely impair the entry of sporozoites into the bloodstream. Overall, data suggest that antibodies targeting the migratory sporozoite exert a large proportion of their protective effect at the inoculation site.69
Dendritic macrophages of the dermis screen the skin for invading microorganisms, then incorporate and process microorganisms. Antibodies directed against the circumsporozoite protein CSP rapidly immobilize sporozoites in vitro. It is likely that these antibodies may be able to act in vivo just after sporozoite deposition into the skin by the mosquitoes and that this could function as a first stage immunity. Intravenous challenge by syringe short-circuits normal sporozoite entry through the skin and misses any immune component contributed by factors in the skin. Sporozoites deposited in avascular skin tissue by mosquito bites are exposed to antibodies for much longer times and they are blocked within minutes if antibodies are present. It should also be noted that the flow of immunoglobulins into cutaneous interstitial tissue is enhanced by the increase in vascular permeability generated by the mosquito bite. And it is possible that duration of protection may be prolonged in populations living in a region in which malaria is endemic, where boosting could theoretically be achieved by repeated exposure with the mosquito bite.
Other authors also found that antibodies severely impair the entry of sporozoites into the bloodstream.70-72 Sporozoites remain up to 2-3 hours in the skin before most of them find the entry into a blood vessel and are very vulnerable at this stage.73,74 This gives ample time for fever to play a role. Fever is a regulated increase of the body temperature resulting from both infectious and non-infectious causes. Fever is known to play a role in modulating immune responses to infection. It was recently demonstrated that febrile temperatures regulate antigen binding of the parasites to antibodies.75 Serum taken from volunteers immune to sporozoite challenge may totally block sporozoite invasion.76
Immunoglobulins protect efficiently by targeting α-gal on sporozoites immediately after inoculation by Anopheles mosquitoes; but not against the disease once the erythrocytic stage of malaria is established. IgE also interferes with the 14-3-3 ε protein during the invasion of hepatocytes by sporozoites. Antibodies are capable of blocking infection of the liver by Plasmodium falciparum. They could block infection at the pre-erythrocytic stage in several ways, either by neutralizing sporozoites directly, opsonizing sporozoites for phagocytosis or blocking invasion of sporozoites into hepatozoites.77-81 Antibodies also exert a protective effect at the level of hepatocytes, at 3 points: sporozoite attachment to the hepatocyte surface, entry, and subsequent cellular development.82 The inhibition of sporozoite’s cell traversal activity seems to be an import element. The immunoglobulin 3D11 for example neutralizes 90% of the sporozoite infectivity by interacting with CSP. Circumsporozoite protein is the antigenic target of RTS,S and of other pre-erythrocytic malaria vaccines currently undergoing clinical trials.83,84 Antibodies reduce motility of sporozoites. In a trial where antibodies of the IgG family were injected the motility was drastically reduced (Figure 2).
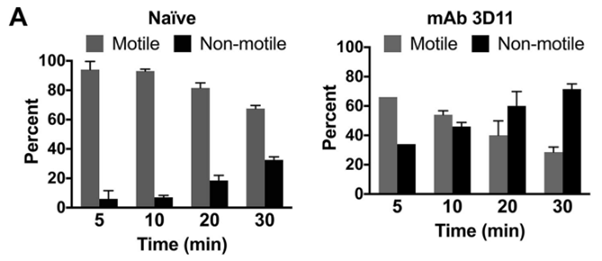
Figure 2 Antibody-mediated protection against plasmodium sporozoites begins at the dermal inoculation site.
Sporozoites inoculated into the skin of mice passively immunized with MAb 3D11 exhibit impaired motility. Mice were passively immunized by i.v. inoculation of 150µg of MAb 3D11. Sixteen hours later, P. berghei mCherry sporozoites were injected intradermally, and their movement in the skin was recorded in 5-min videos and analyzed. (A) Motile and nonmotile sporozoites were manually counted at the indicated time points after sporozoite inoculation, and shown are the percentages of motile and nonmotile sporozoites in MAb 3D11-immunized and naive control mice at each time point. At least 100 sporozoites were imaged per movie per time point. Results from 2-time courses were pooled, and shown is the mean ± standard deviation. The proportions of motile to nonmotile sporozoites between naive and MAb 3D11 inoculated mice were statistically significantly different at all time points.85
A study in Burkina Faso showed a positive association with sporozoite gliding motility inhibition. In vitro hepatocyte invasion was inhibited by naturally acquired antibodies and there were positive correlations between invasion inhibition and gliding inhibition (Figure 3).86
Figure 3 Functional antibodies against Plasmodium falciparum sporozoites are associated with a longer time to qPCR-detected infection among schoolchildren in Burkina Faso.
Antibodies in children from Burkina Faso
It seems thus well established that anti-sporozoite antibodies reduce motility and block the entry of sporozoites into target cells. Subsequent intravital studies revealed in vivo inhibition by antibodies of sporozoite invasion of dermal blood vessels. Anti-sporozoite antibodies act even earlier during challenge by mosquito bite, by inhibiting release of sporozoite from the mosquito proboscis into the skin. Analysis of kinetics of Plasmodium berghei sporozoites injected by mosquitoes into immunized vs non-immunized mice showed significantly fewer sporozoites were deposited in immune mice.87-89 Human sweat contains lactoferrin, a potent killer of protozoans.90-92
Mosquito bites and saliva
For their blood meal mosquitoes inject saliva. The effect of saliva specific adaptive responses in the skin on the infectivity of inoculated sporozoites has not been studied in great detail. Reactions to mosquito bites, being immunological in nature, lead to swelling, wheal and flare of the skin. They are due to the mosquito salivary proteins. Mosquito saliva contains many biological materials, anticlotting and antiplatelet factors and vasodilators which presumably increase the speed at which blood from the host is imbibed. But also, immunomodulators, allergens which bind to IgE and induce histamine and iNOS release. Sporozoites express α-gal (galactose-alpha-1,3-galactose), and the bite of mosquitoes like the bite of ticks may lead to an overload with IgE antibodies. The molecule α-gal is also present on Trypanosoma and Leishmania parasites.93-99
Allergens are present in the saliva of most of the mosquitoes, even in those which are not infected. A study has shown in a murine model that bites from uninfected mosquitoes prior to Plasmodium yoelii infection influence the local and systemic immune responses and limit parasite development within the host. The difference in liver parasite burdens becomes evident at 20 hours post infection. Another strange way to achieve vaccination! And it may explain why people living in countries with dense Anopheles populations are immunized by these bites. The IgE related immunity does not decrease because people are continuously bitten by mosquitoes with the concomitant injection of saliva. Although most of these people are asymptomatic, they do not develop high levels of parasitemia and fever because the saliva of the mosquitoes maintains a high level of IgE. There are also seasonal increases in IgE, after the rainy season. They are absent or low in the absence of bites (Figure 4).100-102
Parasite burden in naïve mice and mice presensitized with A stephensi saliva bites. But an essential question remains to be elucidated. Is it the saliva alone which triggers immunity, or do sporozoites need to be present in the saliva? Immunization by the bite of irradiated, sporozoite infected mosquitoes has become the gold standard for immunization and the assumption that this is entirely related to the immune stimulus of these irradiated sporozoites associated with retardation of development of parasites within hepatocytes.103 Although the mechanism has yet to be completely elucidated, a similar phenomenon has been noticed: repeated infestation with Ixodes scapularis ticks induces resistance to Borrelia burgdorferi transmission. And multiple exposure to bites from uninfected sand flies prior to infection confer resistance to Leishmania major.104,105 And the treatment with leeches is also used for various therapies, probably related to their saliva.106 But let’s stay humble: we are far from developing an efficient vaccine. A paper from 2002 is still valid as quote in 2019:” The incredibly smart and evasive Plasmodium falciparum continues to laugh at our efforts to develop a malaria vaccine”.
The role of Artemisias
The prophylactic efficiency of Artemisia annua was first discovered and documented by Patrick Ogwang in several papers.107 In a Chinese trial a significant boosting of IgGs was noticed by the administration of the aqueous extract of Artemisia rupestris.108 IgE elevations are the expression of CD4+ cells and we have been able to demonstrate that these are increased by the administration of Artemisia annua and Artemisia afra. CD4+ cells are already induced in the pre-erythrocytic stages of malaria. This leads to a wide range of antibodies including some specific against the circumsporozoite protein (CSP).109,110
This is in line with information received from Patrick Ogwang, Mbarara University. That Artemisia annua drinking raises IGs. That, when he drunk tea the day before the biting, a swelling is noticed at the site, where the mosquito had bitten. An inflammatory reaction of course, but as a protective reaction against the invading sporozoites enhanced by the IGs resulting from Artemisia. But this in contradiction with the personal information received from a Belgian lady which states that her allergy against insect bites has diminished since she is drinking Artemisia infusions. Some people apply wormwood directly to the skin for alleviating insect bites.
Polyunsaturated fatty acids (PUFA) play a complex role in inflammation. Gamma-linolenic acid apparently decreases inflammation and blocks amplification of IL-1 by keratinocytes. Arachidonic acid increases it and Artemisia plants are among the plants richest in arachidonic acid.111-113 Aqueous extracts from leaves or seeds of Artemisia annua have a lymphoproliferative effect producing CD4⁺ and CD8⁺ cells, of phenotypes signifying induction of immunological memory.114 It is strange that Artemisia vulgaris which has no known antimalarial properties also raises blood immunoglobulins, CD4, CD8 and lymphocytes. This was well described in a recent Chinese paper. The effect is dose dependent and long lasting (Table 3).115
|
Item |
Artemisia vulgaris meal (96) |
SEM |
P-value |
||||
|
0 |
3 |
6 |
9 |
linear |
quadratic |
||
|
Serum immunoglobulins (g/1) |
|||||||
|
IgA |
0.26c |
0.35b |
0.38a |
0.39a |
0.012 |
<0.001 |
<0.001 |
|
IgM |
0.20c |
0.28b |
0.31a |
0.31a |
0.01 |
<0.001 |
<0.001 |
|
IgG |
0.2 |
0.21 |
0.22 |
0.22 |
0.004 |
0.017 |
0.448 |
|
Whole blood lymphocytes and subsets (%) |
|||||||
|
B lymphocytes |
13.85d |
19.01c |
20.76b |
22.23a |
0.684 |
<0.001 |
<0.001 |
|
T lymphocytes |
30.15c |
35.44b |
3S.77b |
39.16a |
0.709 |
<0.001 |
0.064 |
|
CD4 |
21.80c |
26.97b |
27.62b |
30.58a |
0.699 |
<0.001 |
0.038 |
|
CD8 |
12.76b |
15.75a |
15.96a |
16.52a |
0.345 |
<0.001 |
0.002 |
|
CD4/CD8 |
1.68 |
1.7 |
1.81 |
1.83 |
0.030 |
0.054 |
0.995 |
Table 3 Effect of Artemisia vulgaris meal on the blood immunoglobulins and lymphocytes of Rex rabbits
Similar raises in immunoglobulins by Artemisia annua leaves have been noticed in animal feeding. On day 42 total immunoglobulin titer raised from 5.25 for the control to 8,00 for the experimental diet.116,117 Artemisia sieberi leaves increase monocytes, Camellia sinensis not.118 Amino-acids in Artemisia plants have barely been studied. The analytical data published by EA Brisibe and J Ferreira date back to 2009. Artemisia annua appears to be rich in arginine at 2.20 g/100 g compared with for example 0,04 g/100 g in grapes. Or with a range going from 0.035 to 0,69 g/100 g 6 wild vegetables in Cameroon. Coffee contains 0.1-1.0 g/100 g.119-122
Among all amino-acids, arginine has the specific ability to enhance NO production. Nitric oxide is a known mediator of parasite killing.123 A recent paper from China shows that arginine supplementation not only reduced parasitemia and prolonged survival of malaria infected mice by raising the NO produced endogenously, but also reduced the numbers of zygotes and oocysts in mosquitoes fed on infected blood containing arginine. This confirms earlier results from China. Nitric oxide inhibits the development of Plasmodium yoelii gametocytes into gametes.124-126 Nitric oxide also has a strong effect on gametocyte infectivity. This was first highlighted in a paper in 1993.127
The high arginine content in Artemisia plants my explain their superiority compared with other plants traditionally used in South Africa to treat malaria in the asexual and sexual stages. The action of NO generated by arginine goes beyond the viability of gametocytes in the human host, it also diminishes the infectivity in the mosquito by reducing or even annihilating the formation of gametes and oocysts.128 And the same mechanism involving NO kills Plasmodium sporozoites in the skin, in lymphocytes and in hepatocytes.129 The same authors find that NO limits Trypanosoma, Plasmodium, and Schistosoma development at all stages of the parasite life cycle. Other plants like Camellia sinensis might even significantly suppress the NO production by L-arginine. For whatever reason, but it is well known that some inhibitors of the production of NO by arginine completely annihilate the therapeutic effect of arginine. In a clinical trial arginine supplementation increased the IgM and IgG titers.130
Ketones also play a prophylactic role. This is well known for artemisia ketone. It is also the case for thujone, a ketone present in Artemisia afra and Artemisia absinthium.131 Artemisias are rich in saponins. Naturally occurring triterpene glycosides (saponins) from Quillaja saponaria have considerable adjuvant activity. Adjuvant functions include stimulation of high levels of antibody to T-dependent and T-independent antigens, induction of mouse IgG1, IgG2b, and IgG2a isotypes, and induction of cytotoxic T lymphocyte responses.132 The adjuvant effect has a long-range effect and IgGs continuously increase during weeks after inoculation.133 The prophylactic properties Artemisia, Papaya, Cocoa could be related to the increase in IgGs by asparaginase.134 A traditional polyherbal remedy against malaria from Odisha, India was evaluated for its potential prophylactic activity using in vitro hepatic cell lines assay and the murine malaria system Plasmodium yoelii yoelii/Anopheles stephensi. In vivo a dose dependent effect was noticed (Figure 5).135
Silica particles (Phytoliths) are present in the glandular trichomes of the Artemisia plants. 0,35% in Artemisia annua, 0,22% in Artemisia absinthium, 0,57%, in Artemisia frigida. The presence of trichomes is ubiquitous in the genus Artemisia. 15 taxa were examined to this effect to be used as taxonomic markers.136-138 Silica nanoparticles have the advantage of a high specific surface area and can therefore guest a considerable amount of drug. It is thus surprising to read that silica nanoparticles are extensively applied for drug delivery. Silica particles are used as conveyors of drugs with low bioavailability. Even for artemether.139 IgG covalently bound on silica surfaces has a high stability, even at low pH or in the presence of detergents and urea.140
Silica may even boost the immune system and stimulate IgE production. This effect is known since 40 years. IgG and IgE titers are significantly higher in exposed workers of the brick industry in Egypt. IgE is probably the most important item in acquired humoral activity against malaria.141-144 A single dose of standard quartz with particle size <5μm is able to stimulate in Balb/c mice the production of IgE and IgG1 antibody.145 Silica particles are retained for over 30 days in the tissues because of the endocytosis by macrophages.146 Compared to all this, the role of artemisininin monotherapy may be different. It may offer rapid recovery and fast parasite clearance, but recrudescence is frequent.147
Resistance to artemisinin and ACTs should ring an alarm bell. Plasmodium chabaudi malaria parasites through a stepwise increase in artesunate dose evolve extremely rapidly slow clearance rates. These slower clearance rates provide fitness advantages to the parasite through increased overall density, recrudescence after treatment and increased transmission potential. Removal of only the susceptible parasites by artesunate treatment led to substantial increases in the densities of resistant parasites.148 It has been documented by many authors that anemia protects against falciparum malaria, and that iron supplements increase susceptibility to clinically significant malaria.149 Regular drinking of Artemisia infusions also reduces the plasma iron content.150
Depriving the sporozoite of a metabolically important source of iron may represent a new approach to the development of antimalarial drugs. Some constituents of Artemisia act as iron chelators. Proanthocyanidins are strong iron chelators and Artemisia plants are rich in condensed tannins.151,152 Sulfur present in Artemisias may also play an important role. H₂S has the reputation to be a toxic gas. But at low concentrations it has beneficial health effects and cures several diseases. Most of the balneary tradition is based on the presence of hydrogen sulfide in some mineral waters. The effect may be related to the precipitation of excess iron in the form of insoluble FeS. In their major analytical work Brisibe and Ferreira (op.cit.) find that Artemisia annua contains 0.3 % of sulfur. But only 0.1 % in the majority of other plant leaves.153
In mammalian cells, there is an endogenous production of H₂S. It is located in iron-sulfur clusters in the mitochondria and readily released when needed. In other words, hydrogen sulfide binds the excess of iron which is detrimental in many diseases, but renders it available when needed. Keratin is important for healthy hair and nails. This sulfur containing protein is also a key structural component of the outer layer of human skin! Keratinocytes are able like macrophages to block and phagocyte intruders like sporozoites.
Artemisia plants also may have an effect on transmission. A paper of a South African research team shows that among 8 medicinal plants Artemisia afra has the lowest IC50 for impairing the development of late stage gametocytes. A very important finding as not many plants have such a significant gametocytocidal effect (Figure 6).154
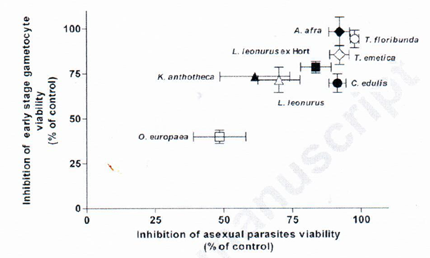
Figure 6 In vitro inhibition of Plasmodium falciparum early and late stage gametocyte viability by extracts from eight traditionally used South African plant species.
Malaria, schistosomiasis and other parasitic diseases
Malaria and helminthic co-infections are frequent. In 2015 a crew of medical doctors in RDCongo under the leadership of Dr Jérôme Munyangi launched a large-scale clinical trial against schistosomiasis using Artemisia annua and Artemisia afra plants. In this study they found that co-infection malaria & helmints are frequent.155
The endemicity of helminthic diseases in sub-Saharian countries entails the development a strong immunity system favoring Th2 rather than Th1.156 Several hypotheses have been advanced on the immune interactions between malaria and helminthiasis and the protective role of IgEs.157,158 Two distinct populations are found for auxiliary T lymphocytes and the profile of cytokines they produce is not the same. Th2 lymphocytes generate mainly Il-4 and IL-5 cytokines which promote the proliferation of B cells responsible for a humoral response. The co-infection malaria-schistosomiasis seems to favor this Th2 and IgE pathway (Figure 7-9). Correlation between helminthic load and serum IgE level helminths and malaria infected individuals.159-161
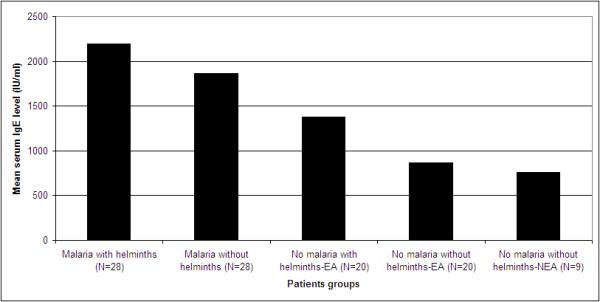
Figure 7 Mean serum IgE levels in adult malaria patients with and without helminths infection and malaria free health controls without and without helminths coinfections. EA, malaria endemic area; NEA, malaria non-endemic area.
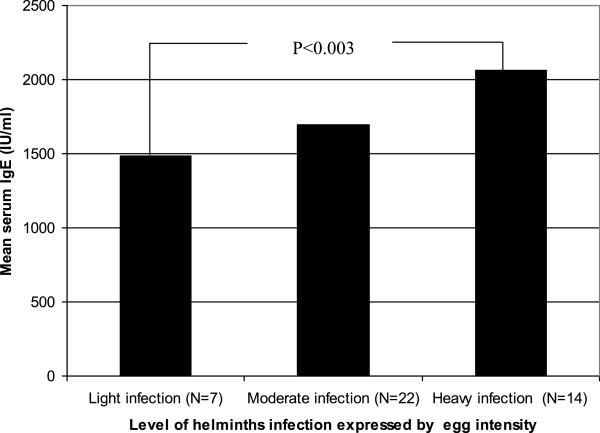
Figure 8 Environmental determinants of total IgE among school children living in the rural Tropics: importance of geohelminth infections.
It was found that immunological pathways involving IgE can lead to damage to the developing schistosomulum and it has been suggested that responses involving IgE could have evolved as protection against helminth infections.162 A French study showed that IgE levels were on average six to eightfold higher in the sera of the majority of adolescents resistant to schistosomiasis. In contrast to IgE, anti-larval IgG and IgM levels were either similar in both groups or higher in the least resistant subjects when these exhibited high reinfection levels.163,164 It is thus not surprising if malaria parasite growth in the liver following sporozoite inoculation is significantly inhibited in mice infected with Schistosoma mansoni and that this results in a large reduction in the percentage of mice that are going to develop blood stage malaria (Figure 10).165
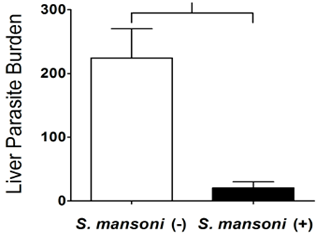
Figure 10 Schistosoma mansoni infection suppresses the growth of Plasmodium yoelii parasites in the liver and reduces gametocyte infectivity to mosquitoes.
Co-infection with helminths seems to protect against severe malaria. This may be tentatively explained by the following hypothesis. The parasitic worm Onchocerciasis volvulus, like Plasmodium falciparum, selectively absorbs and concentrates vitamin A, such that the concentration in O. volvulus is about eight times higher than that in the surrounding tissues of the host. O. volvulus reduces the availability of vitamin A for the malaria parasite in the early sporozoite or blood stage of the lifecycle, which starves and weakens the parasite, perhaps reducing the number of parasites reaching the liver and thereby lessening symptom severity.166,167 A study run in Spain on 370 sub-Saharan migrants showed that 8.9% had submicroscopic malaria (SMM), an occult malaria reservoir in countries to which it is not endemic. A surprising fact of this study was that IgEs were much higher in patients with filarial co-infection. This might exert some protective effect against malaria (Figure 11).168
A striking finding is the relative freedom from malaria in children of Anjouan but not of Grande Comoro, two neighboring islands of the Comoro group in the Indian Ocean. Compared with those of Grande Comoro, Anjouan children were heavily infested with Ascaris lumbricoides.169,170 A recent review paper shows that the effect is not the same for all helminthic infections and that there is some controversy. The main finding is that there is a trend toward a protective effect of Ascaris lumbricoides and Schistosoma haematobium, and worsening effect of hookworm and Schistosoma mansoni on the pathogenesis and incidence of malaria, respectively. It is important however to put emphasis on the general protective effect of helminth infection against severe malaria.171 Other parasitic diseases like sleeping sickness (Trypanosoma brucei) also protect against malaria. Mice were initially infected with T. brucei, followed by administration of P. berghei sporozoites. We observed that a primary infection by T. brucei significantly attenuates a subsequent infection by the malaria parasite, protecting mice from experimental cerebral malaria and prolonging host survival. It reduces a subsequent liver infection by P. berghei. Trypanosoma brucei also produces an array of antibodies (Figure 12).172,173

Figure 12 T brucei infection protects mice from malaria and increases survival (green line Tb/Pb: mice infected 5 days earlier with T brucei).
The problem of travelers
It happens that Artemisia infusions are less efficient for non-immune Caucasians. It is probably not related to genetic strains, but to the absence of acquired immunity. In sub-Saharan Africa most people are almost continuously infected by Plasmodium falciparum parasites, and the majority of infected adults rarely experience overt disease. In naïve individuals Plasmodium falciparum infection is almost always symptomatic, and clinical symptoms can be observed at very low parasitemia levels.174 A study involving several African ethnic groups, some of Caucasian ascent, others of the Negroid type, was unable to detect genetic factors able to explain the significant differences in immune response.175 But in an Indian study, no circulating free antibodies were detected in some individuals. The significance of this trait present in some individuals deserves to be studied in depth.176 The low titer of IgE seems to be persistent if Caucasians live as expatriates in tropical countries the mean level of serum IgE in Nigerian blood donors is significantly higher than those in expatriates living in Nigeria and in Swiss blood donors.177
A serological study from Portugal shows, that 40.29% of travelers with a possible history of malaria exposure were positive for anti-Plasmodium spp. antibodies, while these individuals were negative by microscopy. A similar antibody test is useful to elucidate malaria exposure in microscopy-negative travelers from endemic countries; and in screening prospective blood donors to avoid transfusion transmitted malaria (Figure 13).178
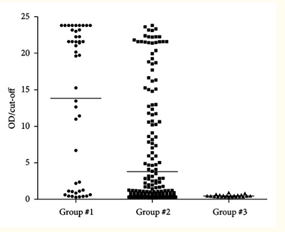
Figure 13 Distribution of antimalarial antibodies in subjects with possible clinical history of imported malaria. Antibody index represents the ratio OD/cut-off for each sample. Group no. 1: travellers potentially exposed to Plasmodium spp. and microscopically positive for malaria (n=45); group no. 2: travellers potentially exposed to Plasmodium spp. and microscopically negative for malaria (n=290); and group no. 3: control, healthy subjects (n=23).
Very interesting is a personal communication from Pamela Weathers: “We have data comparing in vitro responses of Plasmodium falciparum to serum of kids and adults who had malaria to those (all adults) who did not. In vitro studies are challenging to match with in vivo so it requires a different set of controls. There seems to be a host interaction. Blood from malaria-naive patients (never had malaria) does not affect parasites. That despite very high artemisinin levels. Blood from infected patients does kill parasites”.
Gametocytes and transmission
An essential element for continuing transmission of Plasmodium falciparum is the availability of mature gametocytes in human peripheral circulation for uptake by mosquitoes. Natural immune responses to circulating gametocytes may play a role in reducing transmission from humans to mosquitoes. Gametocytes of Plasmodium falciparum go through five (I-V) stages for their development, which under the microscope have different shapes and motilities, those of stage V with their sickle shape having the highest motility. During stage II to V gametocytes hide in the spleen and in the bone marrow for their development.179,180
Recent evidence suggests that the antibodies which intervene during the sporozoite invasion, the merozoite multiplication and the development of immature stage I-IV gametocytes are not the same as those active for stage V mature gametocytes. Antibody recognition was evaluated in a cohort of Ghanaian school children by flow cytometry. The findings support the existence of antigens on the surface of a sub-population of mature gametocyte-infected erythrocytes which induce specific antibody responses. Children with asymptomatic malaria carry antibodies that recognize antigens on the surface of in vitro-cultured erythrocytes infected with mature Plasmodium falciparum gametocytes. These antibodies did not recognize immature gametocyte-infected erythrocytes. The level of recognition varied among individuals. But gametocytes of East African origin can be recognized by antibodies from West African individuals.181-183
Late stage mature gametocytes apparently accumulate in the skin, where they are vulnerable to immunoglobulins in the same way as sporozoites. A study from 1952 run by Belgians in the Democratic Republic of Congo is often cited in this respect. The prevalence of gametocytes in skin samples was 3-fold higher than that in thick smears from finger-prick samples from the same area. In direct skin feeding trials, there were indications that gametocyte densities in the subdermal vasculature was higher than in deeper capillaries. Even individuals without asexual parasites or gametocytes by microscopy can be infectious to mosquitoes. 184-187
The accumulation of parasites in the skin has also been documented for other diseases, like leishmaniasis, trypanosomes, filariasis.188-190
Also mast cells originate from a bone marrow progenitor and subsequently develop different phenotype characteristics locally in tissues. Mast cells play an important protective role, are involved in wound healing, immune tolerance, defense against pathogens and blood-brain barrier functions. These cells are known to accumulate at sites of inflammation in response to parasite and bacterial infections. There they degranulate and set free histamines, IgE and TNF-alpha. Degranulation is proportional to parasitemia, increasing from virtually 0 to 40% in the case of complicated malaria. It is difficult to understand why gametocytes hide in the bone marrow for their development. Mast cells express a high affinity for IgE. Often mast cells are coated with IgE.191-196
Artesunate however is antagonistic with the formation of IgE and might promote transmission.197,198 A paper from Mali is alarming. Artesunate did not clear mature gametocytes during treatment and did not prevent the appearance of new stage V gametocytes as assessed by light microscopy. Baseline gametocyte carriage was significantly higher 6 years after the deployment of artemisinin-based combination therapies in this setting. What worries the authors of the 2016 study from Mali is not only that similar results had been found in a study in 2002-2004, but the fact that baseline gametocyte carriage was significantly higher 6 years later. If artemisinin derivatives really enhance recrudescence and gametocyte carriage, this is indeed alarming. It would mean that ACTs will not eradicate malaria but enhance it in the long run.199
This was confirmed by more recent studies. Mosquito feeding assays showed that artemether-lumefantrine and artesunate-amodiaquine significantly increased gametocyte infectivity to Anopheles gambiae.200 Worse even, a 5-fold increase in gametocytogenesis in Plasmodium falciparum has been documented for chloroquine in vitro.201 It is shocking to read in a recent paper that while chloroquine may significantly reduce mortality, but whether it will interfere with the host immune system is currently unknown. And the authors demonstrate in a mice model that a single dose of chloroquine soon after malaria infection significantly suppresses both the cellular and humoral immunity of the host. The authors conclude that chloroquine only is efficient in the well-established erythrocytic stage by inhibiting hemozoin formation, but, if used in prophylaxis, may have dramatic impacts on the immune system and malaria prevalence.202 This is not surprising as chloroquine reduces CD4+ activation.203
Immunoglobulins and alcohol
There is also the embarrassing question on the influence of alcohol intake. We became alerted to this topic in a large-scale clinical trial with malaria infected patients in RD Congo, comparing ACTs with Artemisia infusion, when we observed a gender difference. Whilst both genders responded equally well to Artemisia, in the ACT-treated arm there was significantly more gametocyte carriage in females than males for days 14-28.204,205 A random survey of 1,190 adults living in a demographic health surveillance site in a malaria endemic area in Kenya was conducted, measuring presence of malaria parasites by slide microscopy. Females were 50% more likely to have malaria than men.206 Having no valid explanation for these observations, one may wonder if it due to differences in enzyme between males and females, like those which are responsible for a different susceptibility of males and females to alcohol consumption. It is well known on the other hand that alcohol consumption, especially of palm wine in Africa, is much higher for males than for females in tropical countries.207
Long-term intake of alcohol affects the immune system. The level of total and specific IgEs in serum is routinely used as tool for the diagnostic of allergies. Several studies have detected an increase of IgEs for people with heavy alcohol consumption.208-211 Serum levels of immunoglobulins (total IgE, IgG, IgM, and IgA) therefore, were analyzed in adult chronic alcoholics in Indian population and were correlated with different epidemiological and alcohol-related parameters. The results showed that 98% of alcoholics had abnormal immunoglobulin levels and 92% showed high or very high total serum IgE levels compared to 24% of the control group. Several other studies have shown that that total serum IgE concentrations are increased in moderate alcohol consumers with respect to abstainers.212 A study from Korea found that the risk of IgE sensitization is significantly higher in male high-risk drinkers.213
Is it dramatic negligence not to have studied in the European Institutes of Tropical Medicine and elsewhere the impact the strong inhibition current antimalarial drugs have on the immune system and might have on prophylaxis and transmission and not to have alerted the African communities against the risks of many pharmaceutical drugs. Chloroquine is still massively sold in Africa and the second most preferred medicine after ACTs. Artemisia tea infusions to the contrary strengthen the immune system, are prophylactic and inhibit transmission. The discovery of the impact of Artemisia tea consumption on the development of specific IgEs could be revolutionary. This finding obviously needs further in-depth studies to be confirmed, and to evaluate other parameters like age, gender, endemicity, simultaneous or previous use of antimalarial drugs. A hypothesis for the beneficial function of IgE antibodies is that they play a key role in very early recognition of foreign material ("gate keeper function") or a general potentiation of the immune system response by improved antigen presentation. Helminths or Plasmodia stimulate a vigorous IgE production, including parasite-specific IgE antibody. Actually, allergy triggered by IgE may provide a beneficial function to the host. Thousands of research papers have been written on artemisinin. But this molecule has no documented effect on sporozoites and gametocytes, on prevention and transmission. Curative and preventive strategies for malaria treatment should ideally target not only one, but three malarial life-cycle stages: exoerythrocytic forms, the asexual blood stages, and the transmission stages. The question often comes up: what is the dosage that should be used for prophylaxis. At this stage of our experience and knowledge the answer is: drinking regularly an aqueous decoction of 5 gr of Artemisia dried herb 3 days per week.
None.
Authors declare that there is no conflict of interest.

©2020 Munyangi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.