eISSN: 2379-6367


Research Article Volume 11 Issue 5
1Central Public Health Laboratory, Ministry of Health, Palestine
2Faculty of Pharmacy, Al-Quds University, Palestine
Correspondence: Saleh Abu-Lafi, Faculty of Pharmacy, Al-Quds University, P.O. Box 20002, Abu-Dies, Palestine, Tel +972-2-2799360
Received: December 18, 2023 | Published: December 28, 2023
Citation: Al-Jaas H, Halayqa A, Jahajha A, et al. Aflatoxin assessment in food commodities: quantification using ICH-validated UPLC-FLD with large flow cell volume. Pharm Pharmacol Int J. 2023;11(5):186-191. DOI: 10.15406/ppij.2023.11.00420
A simple, highly sensitive and fast reversed phase ultra-performance liquid chromatography coupled to a fluorescence detector (UPLC-FLD) method was developed and validated in-house to determine aflatoxins (AFTs) in different food commodities. All the analyses were performed without the need for pre- or post-column derivatization, using a fluorescent large volume flow cell. The separation was achieved on reversed phase Acquity UPLC BEH-C18, 2.1 x 100 mm, 1.7 μm column. The optimal mobile phase consisted of a trinary solvent mixture of acidic water (1% acetic acid), methanol and acetonitrile in a ratio of 540:180:180 (v/v/v) at 25°C. The mobile phase flow rate was fixed at 300 µl/min. The total run time for the separation of the four AFTs was 7 minutes, with an elution order of G2<G1<B2<B1. The method was evaluated for system suitability, specificity, sensitivity, linearity, accuracy (recovery), precision and robustness. All the results were within the allowed specifications of the International Council for Harmonisation (ICH) guidelines. The LOD values were 3.81, 2.66, 6.74, and 3.63 pg/g for B1, B2, G1 and G2 respectively with a relative standard deviation (RSD) of less than 2%. These highly sensitive results are suitable for rapid routine quantitative determination of AFTs in various food commodities at levels of pg/g. The method was applied to simultaneously determine the occurrence of AFTs in 45 samples including spices (n=14), flour (n=3), semolina (n=1), seeds (n=4), powdered milk (n=1), dates (n=13) and thymes (n=9) from local markets. The results meet the maximum allowed limits set by the Palestinian standard institution (PSI) and the European commission (EC). All the samples were first passed through an immunoaffinity column for purification and enrichment, followed by a semi-quantitative test on commercial AFTs kit. Subsequently, the positive samples were quantitatively determined using the validated UPLC-FLD method. It is recommended to exercise care when using commercial kits for AFT testing, particularly if spices or thymes contain colored additives. This precaution is essential to avoid potential inaccuracies that could lead to false-positive results.
Keywords: aflatoxins, ICH validation, UPLC-FLD, immunoaffinity column, food commodities, food contaminants
UPLC-FLD, ultra-performance liquid chromatography-fluorescence detector, AFTs, aflatoxins, ICH, International Council for Harmonisation, PSI, Palestinian Standard Institution, EC, European commission, AOAC, Association of Official Analytical Chemists, lex, fluorescence excitation wavelength, lem, fluorescence emission wavelength, MeOH, methanol, ACN, acetonitrile, H2O, water, NaCl, sodium chloride, PBS, phosphate buffer saline, HOAC, acetic acid, k', capacity factor, Rs, resolution, Tf, USP tailing factor, N, number of theoretical plates, RSD, relative standard deviation, LOD, limit of detection, LOQ, Limit of quantitation, CPHL, central public health laboratory
Aflatoxins (AFTs) are toxic secondary metabolites produced primarily by two fungi, Aspergillus flavus and Aspergillus parasiticus.1 Figure 1 depicts the chemical structure of the four majors AFTs: B1, B2, G1 and G2.
These fat-soluble toxins contaminate a wide range of food matrices, including herbs, spices, nuts, oil seeds, flour, and dates.1 When consumed at high levels, they can have severe effects on the liver and can induce human carcinogenesis.2 In many developing countries, AFTs are considered a significant health risk to both humans and animals.3 Therefore, the separation and analysis of aflatoxins from food and feed samples is crucial for ensuring food safety and compliance with regulatory limits.
In Palestine, there have been limited investigations into the levels of AFTs in food. Only AFTs in chickpeas, multifloral honey, and raw milk have been reported.4,5 Therefore, there is an immediate need for an accurate, versatile, sensitive, and rapid validated analytical method to determine AFT levels in accordance with the PSI and EC specifications.
Various analytical methods are used to analyze AFTs, such as TLC, HPLC coupled with fluorescence detection, and LC-MS.6-13 Waters Corporation has demonstrated three application notes on a method to determine AFTs without derivatization, but these methods have not been validated.10,11,13 Rapid semi-quantitative detection kits based on immunoenzymatic reactions (ELISA) are still commonly used for total AFT determination.14 The official methods by the Association of Official Analytical Chemists (AOAC) rely on immunoaffinity column clean-ups followed by HPLC with detection of AFTs' natural fluorescence or by pre- and post-column derivatization.15,16 The UPLC methodology utilizes columns with sub-2μm particle size, enabling smaller flow rates, superior speed, resolution, and sensitivity.16 Additionally, the use of a large volume flow cell in the fluorescence detector expedites the determination without derivatization.
In Palestine, the levels of AFTs in food commodities pose a significant challenge, particularly in terms of regulation and public health. This study is the first to report on the occurrence of B1, B2, G1, and G2 AFTs in selected food commodities from local Palestinian markets without derivatization. The AFT levels were determined using an in-house validated UPLC-FLD method after subjecting the samples to an immunoaffinity monoclonal column for selective clean-up and recovery of AFTs. The results were compared with the maximum allowed limits adopted by the PSI and EC specifications. The in-house UPLC-FLD optimized chromatographic conditions were validated in accordance with the ICH guidelines.17
Chemicals and reagents
A mixture of aflatoxins (AFTs) reference standards, including B1, B2, G1, and G2 dissolved in methanol, was procured from Supelco (USA). Glacial acetic acid (HOAC) was obtained from Merck (Germany). HPLC-grade acetonitrile (ACN) and methanol (MeOH) were purchased from Sigma-Aldrich (USA). High-purity water was prepared using a Millipore Milli-Q Integral 10 water purification system. Sodium chloride (NaCl) was sourced from Merck (Germany), and Phosphate Buffer Saline (PBS) solution was acquired from Calbiochem (USA). Millex Nylon membrane disposable filters (33 mm, 0.45 µm) were obtained from Merck Millipore, Germany.
Food commodities samples
A total of 45 food commodity samples including spices (n=14), flour (n=3), semolina (n=1), seeds (n=4), powdered milk (n=1), dates (n=13) and thymes (n=9) were randomly collected from local markets in the West Bank by the Environmental Health Department, Ministry of Health, Ramallah, Palestine.
UPLC-FLD system
The Waters Acquity UPLC Fluorescence (FLR) Detector with a 50μl volume flow cell and Empower 3 software were utilized (USA).
Chromatographic conditions
The chromatographic column used was Acquity UPLC BEH-C18, 2.1 x 100 mm and 1.7 μm particle size (Waters, USA). The optimal mobile phase was prepared by mixing the highly purified acidic water (1% acetic acid) with methanol and acetonitrile in 540:180:180; (v/v/v), and allowed to equilibrate to room temperature. The mobile phase was filtered via 0.45 mm cellulose nitrate filter, (Satorius stedim Biotech, Germany) and was degassed by sonication prior to use. The flow rate used was 0.3 ml/minute. The fluorescence wavelength of excitation was 362 nm for all AFTs, and the wavelength of emissions was 429 nm for B1, B2 and 455 nm for G1, G2. The injection volume was 20 ml by using extension loop of 50 μl and the temperature of the column was at 25oC while the vial temperature was 4oC to avoid any deterioration of AFTs. The total run time was 7 minutes with an elution order of G2<G1<B2<B1.
Preparation of standard solutions
From the total AFTs standard stock methanol solution (2466 ng/g), comprising B1 (990 ng/g), B2 (306 ng/g), G1 (870 ng/g) and G2 (300 ng/g), an intermediate total AFTs standard of (24.66 ng/g) was prepared by proper dilution with methanol. Finally, the intermediate solution was further diluted with 1% acetic acid to obtain a total AFTs working standards of 29.59, 59.18, 118.37, 641.16 and 1233.00 pg/g respectively. This solution was used to construct the calibration curve of the four AFTs.
Optimized procedure of AFTs sample extraction and clean up
The sample preparation method used in this study was primarily based on a procedure reported in the ALFAPREP immunoaffinity column leaflet of R-Biopharm Rhone Ltd and by an AOAC method.14,16 However, a modification was made in the final step of the procedure where 1% acetic acid eluent was used instead of pure water.
The validated UPLC-FLD method was developed with the aim of simultaneously resolving the four AFTs (B1, B2, G1, and G2) from each other and from the complex backgrounds of various food matrices. This was done to meet the system suitability test requirements outlined in the ICH guidelines. A mobile phase consisting of 1% acetic acid, methanol, and acetonitrile in a ratio of 54:18:18 (v/v/v) was employed to achieve optimal conditions at a flow rate of 300 µL. The elution time was set at 7 minutes with an elution order of G2 < G1 < B2 < B1. The excitation fluorescence wavelength for all AFTs was 362 nm, while emission wavelengths were 429 nm for B1, B2, and 455 nm for G1, G2. Figure 2 displays a typical UPLC-FLD chromatogram of the four AFTs in a standard mixture under optimized conditions.

Figure 2 UPLC-FLD chromatograms of a standard solution containing AFTs mixture of B1, B21, G1 and G2. A, Chromatogram of G1 and G2 monitored at lex 362 nm and lem 429 nm; B, Chromatogram of B1 and B2 monitored at lex 362 nm and lem 455 nm.
Method validation
The UPLC-FLD method's chromatographic conditions were validated according to ICH guidelines, covering system suitability, specificity, linearity, range, accuracy (recovery), precision (repeatability and intermediate precision), and robustness.
System suitability
The system suitability was assessed by six successive replicate injections of the four AFTs standards of B1, B2, G1 and G2 solution followed by calculating their corresponding peak area, capacity (k'), resolution (Rs), USP tailing factor (Tf) and the number of theoretical plates (N). The total AFTs concentration of the standard solution mixture was 118.36 pg/g as shown in table 1. The UPLC-FLD method met the accepted requirements.
Parameter |
B1 |
B2 |
G1 |
G2 |
Accepted Limits |
% RSD |
0.36 |
0.36 |
0.37 |
0.39 |
£ 2.0% |
Tailing factor (Tf) |
1.01 |
1.07 |
1.15 |
1.11 |
£ 2.0 |
Resolution (Rs) |
3.87 |
2.12 |
3.21 |
≥ 2.0 |
|
Theoretical plates (N) |
44338.5 |
4788.5 |
3898.3 |
5486.6 |
≥ 3000 |
Capacity factor (k') |
5.29 |
3.9 |
3.42 |
2.5 |
≥ 2.0 |
Table 1 Summary of System Suitability for Aflatoxins (AFTs). The overall concentration of the standard mixture is 118.36 pg/g
Specificity
AFTs standard mixture and red pepper flakes sample test solutions were recorded at the same wavelength of excitation (the same for all AFTs at lex 362 nm) and emission (G1 and G2 monitored at lem 429 nm, B1 and B2 monitored at lem 455 nm) in order to assess the specificity of the optimized UPLC-FLD method. The peaks of B1 (5.5 minutes), B2 (4.3 minutes), G1 (3.9 minutes), and G2 (3.1 minutes) in the sample solution precisely coincide with those in the standard solution, indicating no interferences. The results demonstrate that the UPLC-FLD method effectively eliminates unwanted matrix-interfering compounds, affirming its suitability for identifying and quantifying AFTs in food commodities.
Linearity and range
Various concentrations of B1, B2, G1, and G2 (comprising 5 concentrations, each with 3 replicates) were introduced, as outlined in table 2. The regression lines exhibited linearity across the tested range, with R2 values exceeding 0.999 for all AFTs. Additionally, it was observed that all four peaks were distinctly separated at consistent retention times, displaying symmetrical peak shapes.
Sensitivity
The UPLC-FLD method sensitivity was assessed by determining the limit of detection (LOD) and Limit of quantitation (LOQ) for B1, B2, G1 and G2, achieved at a signal-to-noise ratio of 3 and 10, respectively. This involved injecting triplicate series of diluted standard solutions with known concentrations. The obtained LOD values were 3.81, 2.66, 6.74, and 3.63 pg/g for B1, B2, G1 and G2, respectively. Correspondingly, the LOQ values were 12.70, 8.86, 22.40, and 12.10 pg/g for B1, B2, G1, and G2, respectively, with a RSD % of less than 2 (table 2).
|
AFT |
Linearity range (pg/g) |
(R2) |
Linear equation |
LOD |
LOQ |
|
B1 |
11.88-475.20 |
0.9998 |
Y = 6.18e+003 X + 1.71e+004 |
3.81 |
12.7 |
|
B2 |
3.97-146.88 |
0.9996 |
Y = 1.38e+004 X + 9.78e+003 |
2.66 |
8.86 |
|
G1 |
10.44-417.60 |
0.9996 |
Y = 2.60e+003 X + 1.97e+003 |
6.74 |
22.4 |
|
G2 |
3.60-144.00 |
0.9992 |
Y = 8.19e+003 X + 1.04e+004 |
3.63 |
12.1 |
Table 2 Regression and limits of detection (LOD), limits of quantitation (LOQ) values for the four aflatoxins (AFTs) (pg/g)
Accuracy (recovery)
Various concentrations of the four AFTs were introduced into a pure date matrix, and the accuracy, as indicated by recovery, was assessed. The average recovery outcomes at such low concentrations ranged from 80-110%, with a RSD % of less than 3% (n = 3), signifying robust stability and adherence to acceptance criteria (refer to table 3). It's noteworthy that the obtained recovery results also met the recovery limits stipulated by the AOAC and Codex standard.18 For instance, the AOAC specifies an allowable recovery range of 75-120% for a 1 ng/g (ppb) toxin level, while the Codex standard permits 70-110% for concentrations ranging from 1-15 ng/g (10 ppb).
AFT |
Amount added (pg/g) |
Amount found (pg/g) |
Average Recovery (%) |
RSD (%) |
B1 |
303.76 |
308.49 |
101.56 |
0.334 |
257.4 |
256.9 |
99.81 |
0.529 |
|
234.75 |
259.27 |
110.45 |
0.371 |
|
B2 |
93.90 |
83.92 |
88.37 |
0.316 |
79.56 |
70.92 |
89.14 |
0.716 |
|
72.55 |
65.68 |
90.52 |
2.408 |
|
G1 |
269.57 |
265.07 |
98.33 |
1.017 |
226.20 |
211.44 |
93.47 |
0.846 |
|
206.28 |
195.83 |
94.93 |
0.857 |
|
G2 |
93.00 |
74.80 |
80.43 |
1.576 |
78.00 |
70.42 |
90.28 |
1.099 |
|
|
71.13 |
63.00 |
88.57 |
1.556 |
Table 3 Average recoveries and relative standard deviation (RSD %) values at three concentration levels (n=3) for spiked B1, B2, G1, and G2 in date clean samples
Acceptable recovery for 1000 pg/g AFTs is between 75-120%
Precision
Repeatability
One laboratory analyst carried out the assay of (B1, B2, G1 and G2) AFTs with six replicates at a total concentration of 118.36 pg/g, using the same analytical equipment on the same day. The repeatability results of the peak areas of the four AFTs; B1, B2, G1 and G2 indicated RSD % values of 0.56%, 0.98%, 1.4% and 2.12% for B1, B2, G1 and G2 respectively.
Intermediate precision (ruggedness)
Two laboratory analysts performed the assay for B1, B2, G1 and G2 AFTs with six replicates at the same concentration (total concentration of 118.36 pg/g) on different days. The results showed RSD % values of 1.84%, 1.96%, 2.22% and 2.38% for B1, B2, G1 and G2, respectively.
Robustness
The developed UPLC-FLD method's robustness is assessed by evaluating its resistance to minor intentional variations in chromatographic operating parameters. These variations involve making deliberate changes to one chromatographic parameter at a time while applying them to a standard AFTs solution mixture (at a total concentration of 118.36 pg/g), as outlined in table 4. The modifications encompass three distinct mobile phases, three flow rates, two batches of columns filled with the same prescribed stationary phase, and three temperatures injected in triplicate. The RSD % values of the peak area indicate that there is no significant alteration in the final assay results for each of the four AFTs despite the considered variations. The average assay results for the four AFTs fall within the range of 96.4% to 103.7%, demonstrating compliance with the acceptable limit of (96.0 to 104.0%).
Parameter |
Average assay % ± RSD, (n=3) |
|||
Flow rate (ml/min) |
B1 |
B2 |
G1 |
G2 |
0.28 |
102.7±2.33 |
103.3±1.06 |
102.5±2.08 |
102.2±1.12 |
0.3 |
101.9±2.21 |
102.1±1.83 |
102.1±1.84 |
101.7±1.92 |
0.32 |
100.2±1.84 |
99.8±1.96 |
101.6±1.69 |
102.1±1.61 |
Temperature (oC) |
||||
23 |
101.3±1.92 |
102.1±2.12 |
101.4±2.35 |
103.2±1.51 |
25 |
100.5±2.71 |
101.4±2.47 |
100.9±1.37 |
100.3±2.54 |
27 |
98.3±2.23 |
97.8±1.86 |
98.9±2.38 |
101.2±2.26 |
Column lot number |
||||
# 0207321531 |
102.9±1.84 |
100.8±1.96 |
100.4±2.38 |
101.6±2.22 |
# 0208322061 |
101.3±0.91 |
102.5±1.44 |
102.8±1.30 |
99.7±1.52 |
% ACN:MeOH:1% HOAC |
||||
17:17:58 |
103.7±0.98 |
102.9±1.21 |
103.2±0.95 |
101.7±1.25 |
18:18:58 |
100.2±1.24 |
101.8±1.58 |
102.8±0.91 |
103.1±1.47 |
19:19:58 |
98.6±2.14 |
99.2±1.77 |
100.3±2.62 |
96.4±0.98 |
Table 4 Robustness test of the UPLC-FLD method on the determination of the four AFTs of B1, B2, G1 and G2
HOAC is acetic acid; the robustness acceptable limit is between 96-104%.
Utilization of the method in food commodities
The investigation into the presence of AFTs in Palestinian food has been limited until recently. Over the past decade, the Palestinian Environmental Health Department has implemented a monitoring protocol to identify AFTs in food commodities. This protocol involves gathering samples from local markets in the West Bank and subjecting them to analysis at the Central Public Health Laboratory (CPHL). The adopted approach at CPHL initially involves checking for the presence of AFTs using an immunoaffinity clean-up column, followed by semi-quantitative analysis using commercial kits. Subsequently, the validated UPLC-FLD method is employed to determine positive samples at very low concentrations (pg/g-ng/g).
In the present study, 45 randomly collected food commodities underwent direct purification and enrichment, followed by semi-quantitative kit analysis and quantitative chromatographic analysis. The samples included spices (n=14), flour (n=3), semolina (n=1), seeds (n=4), powdered milk (n=1), dates (n=13) and thymes (n=9). Tables 5-9 present the semi-quantitative kit results, as well as individual and total AFT levels using the in-house validated UPLC-FLD method. The results of the spices revealed that red pepper flakes contained the highest AFT levels (2535 pg/g) among all tested samples (table 5).
Food Product |
kit† |
B2 |
B1 |
G2 |
G1 |
Total AFTs |
Red pepper flakes |
(+) |
126 |
2117 |
2.71 |
289 |
2535 |
Sweet red pepper |
(+) |
287 |
1273.8 |
5.8 |
ndǂ |
1566.5 |
Mixed spices mixture |
(+) |
1.5 |
11.37 |
nd |
nd |
12.87 |
Grinded black pepper #1 |
(-) |
nd |
7.5 |
nd |
nd |
7.5 |
Grinded black pepper #2 |
(-) |
nd |
nd |
nd |
nd |
nd |
Grinded black pepper #3 |
(-) |
nd |
nd |
nd |
nd |
nd |
Blended Pizza spices |
(++) |
97 |
1843 |
nd |
nd |
1940 |
Cumin #1 |
(++) |
nd |
nd |
nd |
nd |
nd |
Cumin #2 |
(-) |
nd |
nd |
nd |
nd |
nd |
Red chicken spice #1 |
(++) |
0.22 |
174.4 |
nd |
nd |
174.6 |
Red chicken spice # 2 |
(++) |
128.55 |
270 |
nd |
41.5 |
440.1 |
Spiced breadcrumbs |
(+) |
nd |
nd |
nd |
nd |
nd |
Sumac |
(-) |
44 |
nd |
486± |
nd |
530 |
Cinnamon |
(-) |
nd |
nd |
nd |
nd |
nd |
Table 5 Semi-quantitative and quantitative analysis of aflatoxins (AFTs) in spices using commercial kits and UPLC-FLD. Concentration levels are expressed in pg/g
†The kit employs a semi-quantitative test: (+) indicates concentrations exceeding 4 ng/g but less than 10 ng/g, (++) denotes concentrations surpassing 10 ng/g but less than 20 ng/g, and (–) signifies concentrations below 4 ng/g.
ǂ nd: not detected.
According to Palestinian Regulation (PS485/1999), no specific maximum allowed levels are stipulated. However, in compliance with Commission Regulation (EU 165/2010), the maximum permissible level for B1 is 5.0 ng/g, and for total aflatoxins, it is 10 ng/g.
This concentration falls well below the maximum allowed limit set by the EC (EU 165/2010) which is 5.0 ng/g for B1 and 10 ng/g for total aflatoxins.20 Notably, the Palestinian standard institution (PS) did not specify maximum levels allowed in spices (PS-485-1991).21 Figure 3 shows the chromatogram of AFTs found in red pepper flakes by using fluorescence large flow cell.
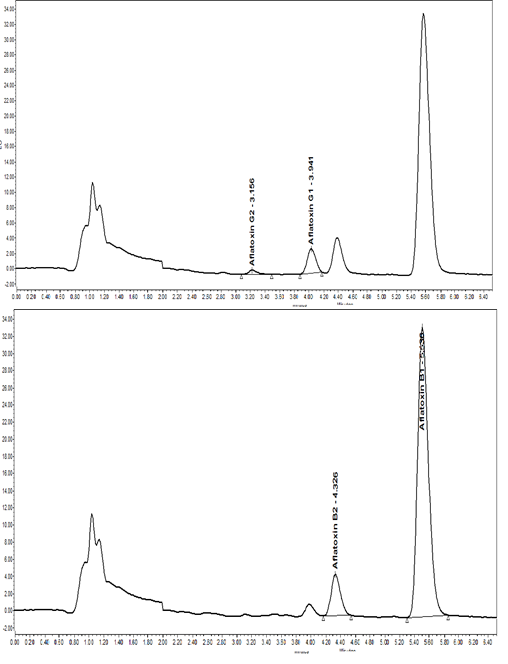
Figure 3 UPLC-FLD chromatograms of AFTs present in red pepper flakes sample (2535 pg/g). A: Chromatogram of G1 and G2 monitored at lex 362 nm and lem 429 nm. B: Chromatogram of B1 and B2 monitored at lex 362 nm and lem 455 nm.
Other samples also showed AFT presence, such as sweet red pepper (1566.5 pg/g), blended pizza spices (1940 pg/g), red chicken (440.1 pg/g) and sumac spices (530 pg/g). Out of 14 samples examined, AFTs in 6 samples gave a negative (-) result using the semi-quantitative kit (equivalent to <4 ng/g AFTs), confirmed by the UPLC-FLD method. However, AFTs in 4 samples were kit positive (+) and 4 samples were kit- (++), equivalent to 4-10 ng/g and 10-20 ng/g AFTs respectively. Surprisingly, when examined using accurate quantitative UPLC-FLD, these 8 kit-positive samples showed results below the kit manufacturer's limit, suggesting a false positive result likely due to the presence of colored additives beyond the kit's capabilities. Flour and semolina samples were found free from AFT residues in both kit and chromatographic results. Similar false-positive results were observed in foods like watermelon seeds, possibly due to added color additives. Powdered milk contained 853.8 pg/g, well below the maximum permissible level set the EC (EU 165/2010).20
Palestinian dates, primarily harvested in Jericho, showed almost no AFTs in both semi-quantitative and UPLC-FLD analyses. Of the 13 date samples examined using UPLC-FLD, two contained G1 contaminant between 9.0-9.6 pg/g, approximately three orders of magnitude below the maximum permissible limit of total aflatoxins (PS 258/2013; EU 165/2010), with G2, B1, and B2 AFTs below the LOD level.20,22 The trace occurrence of G1 residue and the absence of other AFTs suggest proper manufacturing and/or storage practices. All the analyzed thyme samples were found to be AFTs-free using the accurate UPLC-FLD method. However, 5 false positive results occurred with the semi-quantitative kit, likely due to the presence of colored additives common in Palestinian thyme. This observation underscores the potential for misleading results when relying on semi-quantitative kits as a screening tool for AFTs in the presence of colored additives in food commodities.
A rapid and highly sensitive UPLC-FLD method was developed and internally validated for the quantitative determination of AFTs (B1, B2, G1, and G2) in food commodities. The method underwent comprehensive evaluation for linearity, precision, system suitability, accuracy, specificity, ruggedness, and robustness, and the results conformed to the specifications outlined in the ICH guidelines. The method's sensitivity, measured in picograms per gram (pg/g), demonstrated significant improvement compared to previously reported methods utilizing pre- or post-column derivatization. Therefore, the proposed method holds promise for adoption in quantitative quality control and routine analysis of AFTs. Notably, the levels of AFT residues in the examined food commodities were well below the maximum limits set by the PSI and EC. However, caution is advised when employing commercial kits for AFT testing, especially in the presence of colored additives in spices or thymes, to prevent potential erroneous false-positive results.
None.
The authors declare that there is no conflict of interest.

©2023 Al-Jaas, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.