eISSN: 2379-6367


Review Article Volume 8 Issue 3
Chemistry Unit, Faculty of Science, Mwenge Catholic University, Tanzania
Correspondence: Gervas E Assey, Senior Lecturer, Mwenge Catholic University, P.O. Box 1226, Moshi, Kilimanjaro, Tanzania, Tel +255 756 640 546
Received: April 17, 2020 | Published: May 12, 2020
Citation: Assey GE, Mgohamwende R. A review of Titanium, Vanadium and Chromium transition metal Schiff base complexes with biological and catalytic activities. Pharm Pharmacol Int J. 2020;8(3):136-146. DOI: 10.15406/ppij.2020.08.00289
Schiff bases are compounds obtained from the condensation reaction of a primary amine and aldehyde or ketone. They can be coordinated with metals especially the transition metals to form Schiff base complexes. The objective of this work is to review the synthesis, biological as well as catalytic activities of both Schiff base ligands and their transition metal complexes of titanium, vanadium as well as chromium. The methodology used for looking for the references were through the internet. Biological activities that were considered in this review were mainly those of antibacterial and antifungal. For the antibacterial, both gram positive and gram negative strain were considered. The results obtained from the study indicated that the Schiff base complexes, Schiff bases as well as metal ions for the metals titanium, vanadium and chromium were used by different researchers to investigate antimicrobial activities against bacteria, fungi as well as anti-tumour activity against breast cancer. The antimicrobial literature studies showed that the metal ions were having lower inhibition capacity than the ligands but the complexes showed the highest inhibition capacity against the microbial. The use of Mono- and dioxide complexes showed anticancer activity against MCF-7 (breast cancer) cells. The cis-Dioxido vanadium (V) complexes were also investigated for catalytic activity on the oxidation of cyclohexane and gave conversion of 12% and selectivity of up to 85%. The paper is divided into five sections which include introduction, methodology used in looking for references, results and discussion, acknowledgements and references. The results obtained from the review show that the Schiff base complexes were more effective when screened as anti-microbial compounds than their ligands. The complexes also showed effectiveness when screened for their catalytic activity on organic chemistry reactions.
Keywords: Schiff base complexes, antimicrobial, catalytic activities
MCF-7, Michigan Cancer Foundation-7 a breast cancer cell; Ti, Titanium; V, Vanadium; VO, Oxo-vanadium; Cr, Chromium; Fe, Iron; Cu, Copper; Co, Cobalt; Mn, Manganese; Zn, Zinc; Cd, Cadmium; Hg, Mercury; Ru, Ruthenium; MOL, metal organic ligands; DPPH, 2,2-diphenyl-1-picrylhydrazyl; MIC, minimum inhibitory concentration; 2MN, 2-methyl-naphthalen; 2MNQ, 2-methyl-1,4-naphthoquinone; L, ligand; SB, Schiff base; IR, Infra-Red; UV, Ultraviolet; FTIR, Fourier transform infrared spectroscopy; H1NMR, proton nuclear magnetic resonance; ESR, electron spin resonance; NF, not found; TON, turn over number
Schiff bases are formed when any primary amine reacts with an aldehyde or ketone under specific conditions.1–9 Studies show that Schiff base ligands together with their complexes have many biological applications ranging from antimicrobial10–13 and catalysis.14 A number of homogeneous catalytic reactions involve Schiff base complexes which have a major role in such reactions as catalysts.15 Vanadium compounds especially oxo-vanadium (IV) have shown to have a high spectrum of antimicrobial especially against highly resistant gram positive and gram negative as well as against fungal pathogens and anti-oxidant.16 The vanadyl (IV) complexes have been used to treat both insulin dependent type-1 and non-insulin dependent type 2.17,18 Mixed ligands complexes play important role in biological activities.19 The VO(IV) complex with Schiff base L derived from 4-aminoantipyridine, 3-hydroxy-4-nitrobenzaldehyde and o-phenylenediamine has been determined structurally to have square-pyramidal geometry around central metal and antimicrobial screening test gave good results in the presence of metal in the ligand system.20
Among the novel Ti (IV) complexes derivatives, which show antibacterial activity can represent a new exciting approach of designing new antibacterial drugs due to dual possibility of both ligand plus metal ion interacting with different steps of the pathogen lifecycle.21 Bi-functional Schiff base complexes of Lewis acid and Lewis base of Ti (IV)/Ti(V) were applied to the catalytic asymmetric cyanosilylation of benzaldehyde and oxidation of sulphides to chiral sulfoxides.22 Transition metal compounds prepared with vanadium and chromium are known for their redox properties and their capacity to catalyse epoxidation reactions.23 Mixed ligand complexes play an important role in biological field as shown in many ways by which enzymes are activated by metal ions.19 Metal organic ligands (MOL) when allowed to react with metal ions result in the formation of mixed metal complexes.24
Quantum mechanical methods have been used to show that the vanadyl complexes which have phenolate, alcoholate, carboxylate especially when these donors join a Schiff base donor, constitute the most stable complexes.25 Schiff base complexes show a broad range of biological activities including antibacterial, antifungal, antiviral, antimalarial, anti-proliferate, anti-inflammatory, anti-cancer, anti-HIV, anti-helminthic and anti-pyretic.6 Also they show excellent catalytic activity in various reactions and in the presence of moisture. Biologically important form of chromium is the trivalent ion, Cr3+ which is required for proper carbohydrate and lipid metabolism in mammals.26
This review summarizes various applications of Schiff bases and their titanium, chromium and vanadium metal complexes including biological activities, oxidation of organic chemistry reactions and catalytic activities.
Search criteria
To carry out this study, require review of documents and literature materials available from online publications, academic domains and official publications that were obtained from internet. The following criteria were used in searching for the studies used in this paper. Studies that have addressed Schiff bases and Schiff base complexes were selected. Studies that have addressed titanium Schiff base complexes with biological activities were selected. Under the category of biological activities, studies that have addressed antimicrobial screening using titanium Schiff base complexes were selected. Publications that have addressed antibacterial and antifungal were also selected. Studies that have addressed vanadium and oxo-vanadium Schiff base complexes with application as antimicrobial or as insulin enhancing agents were selected. Publications that addressed the ability of chromium Schiff-base complexes as anti-microbial and as catalysts to organic chemistry reactions were selected.
Terms used in the search
The terms used in the search were: ‘Schiff bases’, ‘Schiff base complexes’, antimicrobial’, ‘antifungal’, ‘anti-bacteria’, ‘anti-proliferate’, ‘anti-tumour agents’, ‘insulin enhancing agents’, ‘titanium (IV) Schiff base complexes’, ‘oxo-vanadium Schiff base complexes’, ‘catalytic activity’, ‘hydroxylation’, ‘epoxidation’, ‘oxidation’, ‘salicylaldehyde’, ‘anti-diabetic agents’, ‘biological activity’, ‘structural characterization’, ‘chromium (III) complexes’, ‘Schiff-base ligands’, ‘catalysis’, ‘oxido-vanadium (V) complexes’, ‘anticancer activity’, ‘VO(IV) complexes’, ‘primary amine’, ‘metal salen complexes’, ‘mono-oxido vanadium (V) complexes’. ‘dioxido-vanadium (V) complexes’, ‘unsymmetrical Schiff-base metal complexes’.
Screened publications
The total number of publications screened was 80. Among the studies identified 6 publications reported on chromium Schiff-base complexes, 12 publications reported on vanadium Schiff-base complexes, 2 publications reported on titanium Schiff base complexes, 13 publications reviewed on titanium, vanadium or chromium Schiff base complexes, 22 publications reported on biological activities on Schiff base complexes, 8 publications reported on catalytic activity of Schiff base complexes, 2 publications reported on Schiff base complexes as analytical chemistry sensors, 1 publication reported on quantum mechanical studies on complexes effective in biological activities.
Application of titanium, vanadium and chromium Schiff base complexes and their ligands as anti-microbial agents
Schiff bases with biological activity
Kassim et al.,27 synthesized two diazine Schiff-base ligands HLa and HLb derived from thiocarbohydrazine and salicylaldehyde derivatives using microwave assisted synthesis approach. The general pathway for the synthesis of the two ligands that they used is shown Figure 1.
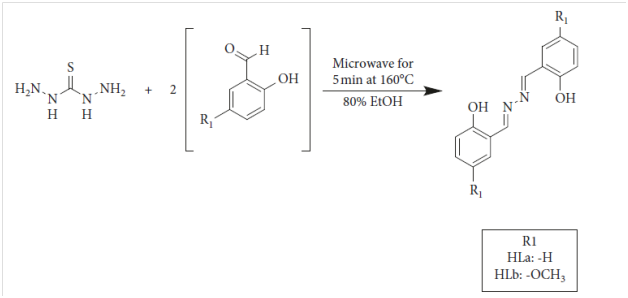
Figure 1 General pathway of synthesizing HLa and HLb ligands.27
The researchers tested the Schiff base ligands against six strains of bacteria namely, B. cereus, B.subtilis, S. aureus, K. pneumoniae, P. auruginosa and E.coli. They showed that HLa was best used against E. coli while HLb does great work on resisting the growth of B. subtilis. They noted that overall ligand HLb had more resistance towards all six bacteria compared to HLa
Titanium Schiff base complexes and their biological activity
Wankhede & Patil28 synthesized and characterized new Schiff-base complex of 1-(1-hydroxynaphthalen-2-yl) ethanone-4-chlorobenzoylhydrazone (H2L) with Ti(III) and Cr(III) metal ions. They screened the Schiff base and metal complexes for their anti-bacterial study using the bacterial cultures of Staphylococcus aureus and Bacillus subtilis for gram positive and Escherichia coli and Salmonella typhi for gram negative. The standard used was that of Penicillin which showed that their anti-microbial activity against E. coli for all metal complexes was more compared to that of penicillin. Also Ti(III) complexes were tested for their anti-fungal activities using fungal cultures of Penicillium chrysogenum, Aspergillus niger, Fusariumoneli forme and Aspergillus flavus. The standard used was Gresiofulvin. Ti(III) complexes exhibited good anti-fungal activities with more than 90% reduction in growth against A niger. The structure of the hydrazine Schiff base is shown below (Figure 2).
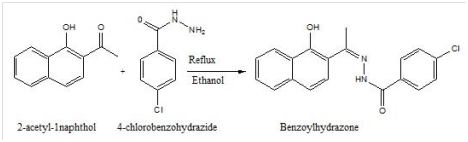
Figure 2 Synthesis of hydrazine Schiff base.28
Ti(IV) complexes of composition [TiCl2(SB)2] were synthesized by reacting TiCl4 and (SBs), where (SBs)=A1(tetracycline hydrochloride Schiff base); B1(streptomycin Schiff base); C1(ceffixime Schiff base) and D1(ampicillin Schiff base) in fixed molar ratio 1:2.21 The synthesized complexes were screened for their anti-microbial activity against ten microbial species. The in-vivo biological evaluation showed that the metal complexes exhibited higher anti-microbial activity than the free ligands. The minimum inhibitory concentration (MIC) values of the Ti(IV) complexes obtained by the researchers are presented in Table 1 below. A comparative study of the ligand and its complexes (MIC values) by the researchers indicated that the complexes showed higher anti-bacterial activity than the free ligand. It was found from the MIC values that the complex [TiCl2(A1)2] was more effective against A. facalis, S. aureus, [TiCl2(B1)2] was more effective against K. pneumonia, [TiCl2(C1)2] was more effective against S. typhimurium and [TiCl2(D1)2] was more effective against S. typhimurium, S. aureus, M. luteus than it was for the other respective bacterial strains.
|
Serial Number |
Microbial Species |
Minimum Inhibitory Concentration (µg/mL) |
|||
|
[TiCl2(A1)2] |
[TiCl2(B1)2] |
[TiCl2(C1)2] |
[TiCl2(D1)2] |
||
|
I. |
S. typhimurium |
62.5 |
250 |
500 |
250 |
|
2 |
B. cerium |
500 |
500 |
1000 |
500 |
|
3 |
S. epidermidis |
500 |
500 |
1000 |
1000 |
|
4 |
A. faecalis |
15.6 |
500 |
1000 |
500 |
|
5 |
S. aureus |
15.6 |
500 |
500 |
250 |
|
6 |
M. lteus |
31.2 |
500 |
1000 |
250 |
|
7 |
A. hydrophia |
31.2 |
31.2 |
500 |
1000 |
|
8 |
K. pneumoniae |
1000 |
62.5 |
500 |
500 |
|
9 |
P. aeroginesa |
125 |
125 |
500 |
1000 |
|
10 |
S. sonnei |
1000 |
1000 |
1000 |
500 |
Table 1 Minimum inhibitory concentration (MIC) values of Schiff bases complexes of titanium (IV)21
Vanadium Schiff base complexes and their biological activities
Pethe et al.,29 synthesized unsymmetrical Schiff-base metal complexes VO(IV) and Cr(III). The Schiff base that was derived from salicylaldehyde (2-hydroxy-3-methoxybenzaldehyde) reacted with ethylenediamine. Both the Schiff base and its complexes were screened for their antibacterial study against E.coli, S abony, S. aureus and B. subtilis. The result showed that the metal complexes VO(IV) and Cr(III) with Schiff base ligands showed bacteriostatic behaviour towards all the bacterial strains more than the Schiff base ligands. Warad et al.,30 synthesized a series of poly-dentate Schiff-base ligands by condensation of H2N-NH-CO-NH-NH2 with various aldehydes and investigated the coordination behaviour of the ligands with metal complexes of type M(acac)xL [M = VO(IV)] where L is the Schiff-base ligands and x = 0 or 2. The biological activities of all isolated ligands and their metal complexes were studied by screening the compounds against microorganisms E. coli, S.aureus, A. niger and C. albicans. The results of their screening showed that 5-methyl substituted vanadium complexes exhibited high activity against E. coli and S. aureus among bacteria, and A. niger and C. albicans among fungus. Ahmed et al.,17 synthesized and characterized some vanadyl Schiff base complexes of neutral tetradentate N2O2 type. They used a Schiff base formed from condensation of o-aminophenol, benzidin and 1,4-phenylenediamine with benzyl saliasldehyde and 2-methylcyclo-pentane-1,3-dione in alcohol media. The researchers characterized all the complexes by use of elemental analysis, melting point, IR, UV-Vis spectra and magnetic moments properties. The UV-Vis spectral data and magnetic moments suggested that all complexes were square pyramidal geometry.
Ebrahimpour et al.,31 synthesized and characterized Mono- and dioxide-vanadium (V) complexes of tridentate ONO Schiff base ligand 1-(((5-chloro-2-oxidophenyl)imino)methyl)naphthalene-2-olate [L2-]. They characterized the complexes using elemental analysis, molar conductivity, FTIR, H1NMR analysis and electronic spectroscopy. The structure of the free ligand H2L and all the complexes were determined by single X-ray diffraction. The title complexes were investigated against MCF-7 (breast cancer) cells and compared with that of VO(acac)2. They obtained results that showed that the complexes possess higher anticancer activities than VO(acac)2.
Savithri & Revanasiddappa16 synthesized and characterized Oxido-vanadium (IV) complexes of 2-(E)-(6-Fluorobenzo[d]thiozol-2-ylimino) methyl)-6-methoxyphenol and screened them for their in-vitro antimicrobial as well as antioxidant activities. The synthesized route of the ligand and its complexes is shown in Figure 3 below.

Figure 3 Synthetic route of ligand HL and its complexes.16
The synthesized compounds showed a broad spectrum of antimicrobial as well as antioxidant activities against a panel of highly resistant gram negative and gram positive bacterial and fungal pathogens which showed that the complexes have better antibiotic action than its parent ligand and standard drugs used. The in-vitro antioxidant activity was determined by DPPH scavenging assay. All the complexes were found to be as good scavenger of DPPH radical comparing to free ligand.
A series of transition metal complexes which include VO (IV) were synthesized from Schiff base (L) derived from 4-aminoantipyrine 3-phenyenediamine.20 Structural characteristics of the obtained complexes were done using elemental analysis, magnetic susceptibility, molar conductance, mass, IR, UV-Vis,1HNMR and ESR spectral studies. The reactions which were used by the researchers for the formation of the Schiff base ligandis shown in Figure 4 below.

Figure 4 Reactions for the formation of shift base ligand.20
From the structural data, the structure of VO (IV) complex was suggested to be of square pyramidal geometry. The proposed structures of the Schiff base complexes are shown in Figure 5, where VO (IV) is among the metal ions used to form the Schiff base complexes. The compounds were tested against the bacteria: Salmonella typhi, staphylococcus aureus, Escherichia coli and Bacillus subtilis using in-vitro biological screening method. The antifungal activities were also done by the researchers by evaluating the compounds against Aspergillus niger, Aspergillus flavus and Rhizoctania bataicola cultured on potato dextrose agar as a medium. All antimicrobial screening test gave good results in the presence of the metal ion in the ligand system.
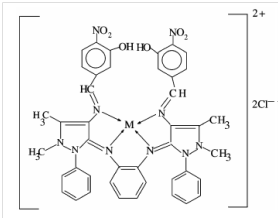
Figure 5 The proposed structures of Schiff base complexes M= Cu(II), Ni(II), CO(II), Mn(II), Zn (II), Cd(II), Hg(II) and VO(IV).20
A Schiff base ligand L was prepared from the condensation of 2-amino-3-benzyloxypyridine and 5-chloro-salicyl-aldehyde.32 The synthesized (L) (E)-2-((3-(benzyloxypyridinylimino) methyl)-4-chlorophenol were reacted with Co(III) and VO(IV). The schematic representation of the synthesized Schiff base ligands together with the obtained complexes from the reactions of VO(IV) and Co(III) are represented in Figures 6 & 7 below:
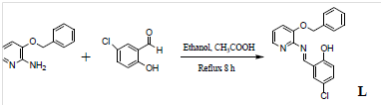
Figure 6 Schematic representation of the synthesis of the Schiff base ligand.32

Figure 7 Proposed structure of the Schiff base complexes.32
The Schiff base complexes were subjected to antibacterial screening for Gram positive bacteria (B. subtilis and S. aureus) and gram negative bacteria (S. typhi and E. coli) antifungal activity against C. albicans and A. niger. The results obtained from their study indicated that all compounds had antimicrobial activity against bacteria and fungi.
Chromium Schiff base complexes and their biological
Chandra & Pipil33 synthesized chromium (III) Schiff base complexes using Schiff base 2,3,9,10 tetraphenyl-1,4,8,11-tetraazacyclotetradeca-1,3,8.10 tetraene. (BDP), 2,4,10,12- tetramethyl 1,5,9,13-tetraazacylohexadeca-1,4,9,12, tetraene(ADP), 2,3,9,10 tetramethyl tetraazatetradeca-1,3,8,10 tetraene (DDP) and tested the compounds using some plant pathogenic bacteraia and fungi. The structures of the ligands BDP, ADP and DDP are showed in Figure 8.
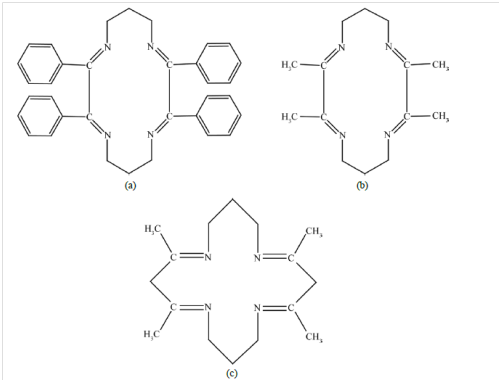
Figure 8 Structures of the ligands (a) BDP; (b) ADP; (c) DDP.33
The ligands (L) 2,3,9,10 tetraphenyl-1,4,8,11 tetraazacyclo-tetradeca-1,3,8,10. Tetraene (BDP), ligands, free metal ions and its complexes were evaluated against different species of bacteria and fungi. The researchers found that in both antibacterial and antifungal studies ligand free metalion in solution showed inhibition capacity slightly less than the ligand but much less than the complexes against all species studied. Anti-fungal screening were done for Aspergillus niger and Aspergillus glaucus fungi while anti-bacterial screening were done against sarcinalutea (Gram positve and Escherichia coli (gram negative).The structures of the metal complexes [Cr(BDP)X2]X, [Cr(ADP)X2]X and [Cr(DDP)X2]X derived from the ligands BDP, ADP and DDP where (X = Cl-, NO3-, NCS-) are shown in Figure 9 below.
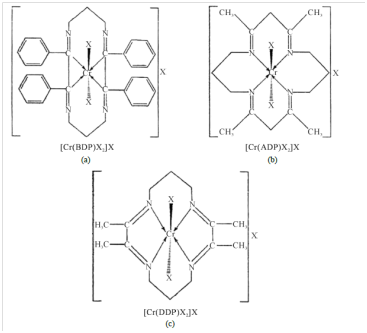
Figure 9 Structures of the complexes.33
Sridhar et al.,10 synthesized metal complexes of copper, nickel, cobalt and chromium from (Z)-4-fluoro–N-(2,7-dimethythept-6-enylidene) benzenamine. The schematic representation of the synthesis of ligand is shown in Figure 10 below.
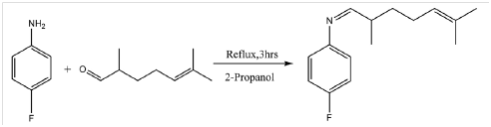
Figure 10 Synthesis of Schiff base ligand.10
The antibacterial and antifungal activity of the Schiff base ligands and the complexes were conducted against three gram negative (E. Coli, S. Typhi and P. aeruginosa) and three gram positive (S.aureus B. subtilis, B. megaterium) bacteria strains and six fungi (C. albicans, P.Chrysogenus A. niger, A. flavus, A. fumigates, C. oxysproum) at various concentrations of 25, 50, 100, 200, 400, 500µg. The minimum inhibition concentration (MIC) was comparable with that of standard drug gentamycin for bacteria and amphotericin for fungi. This is shown in Tables 2 & 3 below.
|
|
MIC (in mm)/µg |
||||||
|
Organism |
Schiff base ligand |
Copper(II) Complex |
Nickel(II) Complex |
Cobalt(II) Complex |
Chromium(III) Complex |
Gentamycin |
|
|
Serial No |
Gram negative |
||||||
|
1 |
E. coli (ETEC) |
800(3) |
NF |
800(5) |
400(5) |
400(3) |
25(18) |
|
2 |
S. typhi |
800(4) |
NF |
800(4) |
800(2) |
800(5) |
25(2) |
|
3 |
P. aeruginosa |
400(4) |
400(2) |
200(2) |
400(3) |
400(3) |
100(1) |
|
Serial No |
Gram positive |
||||||
|
1 |
S. aures |
400(3) |
800(2) |
800(6) |
400(4) |
400(5) |
25(13) |
|
2 |
B. subtilis |
800(2) |
NF |
NF |
200(2) |
NF |
25(8) |
|
3 |
B. megaterium |
800(5) |
NF |
800(4) |
800(4) |
800(3) |
25(7) |
Table 2 Anti-bacteria activity of Schiff base ligand and metal complexes copper(II), nickel(II) Cobalt(II) and Chromium(III)10
MIC, lowest concentration of drug that inhibits growth of the pathogen; NF, MIC not found in the concentration screened
|
|
Organism |
MIC (in mm)/µg |
|||||
|
Schiff base ligand |
Copper(II) Complex |
Nickel(II) Complex |
Cobalt(II) Complex |
Chromium(III) Complex |
Amphotericin |
||
|
Serial No |
fungi |
||||||
|
1 |
C. albicans |
NF |
NF |
NF |
NF |
400(4) |
50(2) |
|
2 |
P. chrysogenum |
800(3) |
NF |
NF |
400(3) |
800(4) |
800(4) |
|
3 |
A. Niger |
800(3) |
NF |
NF |
800(2) |
NF |
100(2) |
|
4 |
A. flavus |
NF |
NF |
NF |
800(4) |
NF |
400(7) |
|
5 |
A. fumigatus |
NF |
NF |
400(7) |
400(3) |
400(2) |
200(2) |
|
6 |
C. oxysproum |
NF |
800(3) |
NF |
NF |
NF |
50(2) |
Table 3 Anti-fungal activity of Schiff base ligand and metal complexes copper(II), nickel(II), cobalt(II) and chromium(III)10
MIC, lowest concentration of drug that inhibits growth of the pathogen; NF, MIC not found in the concentration screened
Complexes of chromium (III) with micro-cyclic ligands -3,4,12,13–tetraphenyl 2,5,11,14 tetraazatricyclo [13:3:1: 16, 0] cosa -1,2,4,6,10 (20) 11,13,14,15 (19) 16 undecane. (BDPY) 3,5,13,15,-tetraphenyl-2,6,12,16 tetraaza tricyclo [15.3.1.17,11] docosa - 1,2,5,7,9,11 (22) 12,15:7 (21), 18-decane (ADPY), 3,4,2,13– tetramethyl-2,5,11,14-tetraaza-tricyclo [13:3.1.16,10] cosa-1,2,4,6,8,10, (20), 11,13,15,(19), 16-decane. (DDPY) were synthesized and tested for their antimicrobial activities.11 The structures of the synthesized ligands and complexes are shown in Figures 11 & 12.
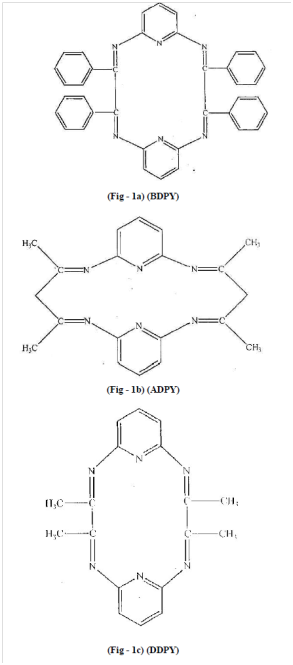
Figure 11 Structures of ligands (Fig. 1a-1c) in.11
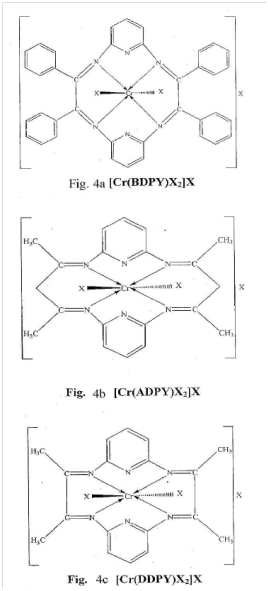
Figure 12 Structures of complexes (Fig. 4a-4c) in.11
The ligands (L) 3,4,12,13-tetraphenyl 2,5,11,14 tetraaza tricycle [13:3:1.16.0] cosa-1,2,4,6,8,10 (20) 11, 13,14, 15 (19), 16 undecane (BDPY). ligand free metal ions and its ligands were evaluated against different species of bacteria and fungi in both, antibacterial and free metal ions in solution showed inhibition capacity slightly more than the ligands but much less than complexes against all species under study. Antibacterial screening was done against Sarcinalutea (gram–positive) and Escherichia coli (gram-negative) antifungal screening were done against Aspergillus–niger and Aspergillus–glaucus.
Catalytic activity
Catalytic activity of vanadium complexes
Silva et al.,34 Synthesized, characterized and tested the catalytic activities of the two novel cis-Dioxo-vanadium (V) complexes: VO2(L)(1) and [VO2(HLOx)](2) and investigated the potential use of the two complexes (1) and (2) as catalysts in oxidative processes of cylo-hexane. The schematic representation of the reaction used to synthesize ligands HL and HLox, [(VO2L](1) and [VO2HLOX] (2) is shown in Figure 13 below.

Figure 13 Synthesis of HL, HLox, [VO2(L)](1) and [VO2(Lox)](2).34
In the investigation of complexes, (1) and (2) as potential catalysts in oxidative processes, their reactivity were investigated through the oxidation of cyclohexane. The schematic representation of the cyclohexane oxidation reaction using the complexes as catalysts is shown in Figure 14.
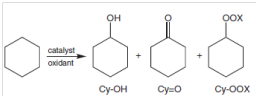
Figure 14 Cyclohexane oxidation products (x=H or t-butyl).34
The products of oxidation include cyclohexanol (Cy-OH), cyclohexanone (Cy=O), cyclohexylhydroperoxide and/or cyclohexyl-tert-butylhydroperoxide (Cu-OO-t-Bu) were detected after 24 h of reaction in the presence of the desired complex and H2O2 or t-BuOOH. Their results are summarized in Table 4 below.
|
Catalyst |
Oxidant |
Product |
Overall conversion |
Turnover |
Selectivity |
|
H2O2 |
Cy-OH |
13% |
|||
|
Cy=O |
12% |
137 |
14% |
||
|
1 |
Cy-OOH |
73% |
|||
|
t-BuOOH |
Cy-OH |
0% |
|||
|
Cy=O |
1.40% |
15 |
14% |
||
|
Cy-OO-t-Bu |
86% |
||||
|
H2O2 |
Cy-OH |
17% |
|||
|
Cy=O |
5.70% |
62 |
12% |
||
|
2 |
Cy-OOH |
71% |
|||
|
t-BuOOH |
Cy-OH |
0% |
|||
|
Cy=O |
2.10% |
23 |
42% |
||
|
|
|
Cy-OO-t-Bu |
|
58% |
|
Table 4 Product distribution for the cyclohexane oxidation after 24 h34
Complexes 1 and 2 presented overall conversions towards the cyclohexane which was between 5.7 and 12% when using oxidant H2O2 having high selectivity of up to 85% to the cyclohexyl hydroperoxide intermediate in mild condition of 1 atm and room temperature.
Catalytic activity of chromium complex
Two poly-dentate Schiff base complexes of Ru(III), Cr(III) and Fe(III) were synthesized and used in the catalytic oxidation of 2-methyl naphthalene (2MN) to 2-methyl-1,4-naphtoquinone; vitamin K3, menadione, 2MNQ using hydrogen peroxide, acetic acid and sulfuric acid.14 The possible reaction mechanism of the ligands and the suggested structures of the Schiff base metal complexes are shown in Figures 14 & 15.
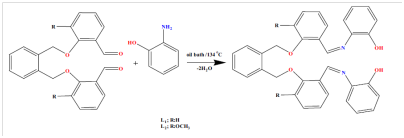
Figure 15 Possible reaction mechanism of the ligand.14
The catalytic oxidation of 2-methyl-naphthalene to 2-methyl-1,4-naphthoquinone (vitamin K3) was done by using the synthesized complexes. The 2MN conversions and the 2MNQ selectivity obtained by the researchers are given in Table 5 below. They dissolved 2MN and complexes in acetonitrile, glacial acetic acid, sulfuric acid (98%) and H2O2 (35%) in the reaction flask and refluxed for 12 h. They used the same conditions for the blank reaction for the purpose of comparison with the reactions using catalysts. The selectivity and conversion for the blank were 3.10% and 29.5% respectively. The selectivity using chromium complex showed 10.11% and 39.14% for L1-Cr(III) respectively. L2-Cr(III) was 10.32% and 40.15% respectively. This showed that, the two chromium complexes exhibited catalytic activity (Figure 16).
|
Catalyst |
Conversion (mol %) |
Selectivity (mol %) |
TON |
|
Blank |
29.5 |
3.1 |
- |
|
L1-Fe(III) |
79.11 |
58.54 |
78.25 |
|
L1-Ru(III) |
63.24 |
32.82 |
62.96 |
|
L1-Cr(III) |
39.14 |
10.11 |
40.26 |
|
L2-Fe(III) |
78.25 |
57.22 |
72.52 |
|
L2-Ru(III) |
61.36 |
35.81 |
58.33 |
|
L2-Cr(III) |
40.15 |
11.32 |
38.16 |
Table 5 2MN conversion and 2MNQ selectivity14

Figure 16 Suggested structures of Schiff base metal complexes.14
This review study have revealed that both free ligand and metal Schiff base complexes of the transition metals of titanium, vanadium and chromium have shown biological activities toward micro-organisms such as bacteria and fungi. The metal complexes showed higher anti-microbial activities than the free ligands and free metal ions. Also the study done on the catalytic activities towards organic chemistry reactions revealed that the metal Schiff base complexes of vanadium and chromium showed catalytic activities towards these reactions.
The authors are grateful to Mwenge Catholic University for support. We also acknowledge the enormous help obtained from the authors whose articles are cited and included in this work.
No conflict of interest.

©2020 Assey, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.