eISSN: 2379-6367


Research Article Volume 4 Issue 7
1Department of Pharmaceutical Sciences, Dr. Hari Singh Gour University, India
2Ravi Shankar College of Pharmacy, India
Correspondence: Vandana Soni, Department of Pharmaceutical Sciences, Dr. Hari Singh Gour Central University, Sagar, Madhya Pradesh, India, Tel +919425172178
Received: October 04, 2016 | Published: December 28, 2016
Citation: Soni V, Sharma VK, Pandey V, et al. A novel strategy for the delivery of doxorubicin to reduce cardio toxicity. Pharm Pharmacol Int J. 2016;4(7):488-493. DOI: 10.15406/ppij.2016.04.00101
Doxorubicin (DOX) is an effective anticancer drug but its effectiveness is limited due to their toxicities after long term use required in cancer chemotherapy which leads to damage of the cardiac muscles and may be irreversible in nature. The approach based on novel drug delivery system gives the new hopes and ray of light to overcome the problems associated with the Dox as well as other anticancer drugs. In present research paper the DOX bearing nano-aggregates (DN) and folic acid conjugated DOX bearing nano-aggregates (FDN) were prepared to increase the therapeutics index of drug by enhancing the drug concentration at target site and avoiding their effect or by passing the healthy organ and cells. In present study PLGA-PEG block polymeric system was synthesized by ring opening polymerization techniques using NHS and then conjugated with DOX. The synthesis of F-PEG-PLGA-DOX was confirmed by 1H-nuclear magnetic resonance (NMR). Prepared nano-aggregates of DOX were studied for particle size, entrapment efficiency, in-vitro and in-vivo studies.
The size of DN was found to be 135±0.6nm whereas slightly increased size i.e. 141±0.8nm was found in case of FDN. The %entrapment efficiency was found to be 79.3±0.5% and 71.7±0.5% of DN and FDN respectively. The in-vitro drug release studies show initially fast release of drug for 10 h. The in-vivo biodistribution studies showed the lesser uptake of DOX in heart in case of nano-aggregates formulation (DN and FDN) as compare to plain DOX after i.v. administration. This may be due to less concentration of free drug available for the absorption by the organ, which indirectly indicated that the drug may be available for targeting to the cancer cells via receptor mediated endocytosis due to the presence of folic acid with the nano-aggregates.
Keywords: nano-aggregates, doxorubicin, cardiotoxicity, cancerous cells, folic acid
DOX, doxorubicin; DN, DOX bearing nano-aggregates; FDN, folic acid conjugated DOX bearing nano-aggregates; FA, folic acid; PLGA, poly(d,l-lactide-co-glycolide); PEG, poly ethylene glycol; NMR, 1h-nuclear magnetic resonance; NHS, n-hydroxy-succimide; DCC, dicyclohexylcarbodiimide; DCM, dichloromethane; DMSO, dimethylsulfoxide; TEA, triethylamine; HPLC, high performance liquid chromatography
Doxorubicin which is also known as Adriamycin is obtained from a mutated strain of the Streptomyces peucetius group. It is well and clinically proven that the drug doxorubicin having the significant therapeutic activity in many cancers types. It became the most widely used Anthracycline and is considered as most potent approved chemotherapeutic drug against cancer.1 With the doxorubicin it is very unfortunate that the plain doxorubicin is not able to target any specific tumor molecular markers for example receptors or growth signal drivers. Doxorubicin also affects the growth and function of many other cell types in the body as well as induced myelosuppression. Although dosage and schedule of doxorubicin affects the severity of side effects.2 Further prolonged treatment with doxorubicin leads cardiomyopathy and heart failure. And now a days the cardiotoxicity, without any doubt is a major issue. A number of approaches have been planned and executed to increase the therapeutic index of doxorubicin, some of which were able to reduce the associated cardiotoxicity. The best example is development of Anthracycline analogs i.e., epirubicin (EpiDox) and Mitoxantrone which are novel analogues that reduced cardiotoxicity, but net therapeutic index of these analogs are very small.3,4 The use of cardio-protective agents such as β-Blockers and angiotensin II receptor blockers (dexrazoxane) also helpful to reduce doxorubicin associated cardiotoxicity. Immunoconjugates, liposomes, Nanoparticles, erythrocyte polymeric micelles and macromolecular conjugates are some of the alternative approach which involves the modification of pharmacokinetics of the drug.5 Such delivery systems can reduce DOX deposition in the heart and in certain cases, may increase the specific accumulation of the dox at desired site. That is why in present time, these drug delivery systems are gaining more attention for effective delivery of anticancerous drugs which can reduce the side effects of conventional drug delivery systems6-8 and enhances the availability of anticancer drugs at target site.9 In this respect, nano-aggregates using amphiphilic macromolecules may be a promising vehicles for antitumor targeting.10 The nano-aggregates are polymer-drug conjugated amphiphilic block as a novel supramolecular colloidal carrier for poorly water-soluble drugs in molecular targeted therapies, with certain advantages such as long retention time, targeting potential, ease of preparation and storage, improved drug solubilization, stability in body fluids and reduced dosing frequency.10,11
In this study, we prepared anticancer agent-incorporated nano aggregates using novel block copolymer. This system having the unique properties, i.e. the hydrophobic domain of the copolymer forms an inner core, and the hydrophilic domain forms a hydrated outer shell. The hydrophobic core is responsible for drug incorporation, and the hydrated outer shell guards the micelle from attack by the reticuloendothelial system.12,13 This core-carona-type block copolymer system are attractive vehicles for solubilizing water insoluble drugs, site specific drug delivery either by active or by passive targeting mechanisms, reducing the dosages of drug, and help to prevent unwanted side effects.
In present study, we synthesized block copolymers composed of PEG and PLGA and then doxorubicin bearing polymeric nano aggregates was prepared. In this system first folic acid was conjugated with the PEG bis amine to make tumor specific drug delivery system. Although anti tumor efficacy studies are in progress. With this paper the results of the reduction in cardiotoxicity of the doxorubicin was shown. In prepared formulation the PEG and PLGA has a hydrophilic and hydrophobic domain, respectively whereas PEG-PLGA block copolymer may showed amphiphilic properties in an aqueous environment. The prepared block copolymer would be able to self-assemble as a core-carona-type polymeric micelle. In addition, the doxorubicin was incorporated with the F-PEG-PLGA block copolymer and evaluated for their in vitro and in vivo effects.
In this study, we prepared anticancer agent-incorporated nano aggregates using novel block copolymer. This system having the unique properties, i.e. the hydrophobic domain of the copolymer forms an inner core, and the hydrophilic domain forms a hydrated outer shell. The hydrophobic core is responsible for drug incorporation, and the hydrated outer shell guards the micelle from attack by the reticuloendothelial system.12,13 In present finding, we aimed to formulate and evaluate the DOX bearing nano-aggregates for the tumour delivery and reducing the cardiotoxicity of the long term use of DOX for cancer treatment which is the main challenge with its use. Although anti tumor efficacy studies are in progress. With this paper the results of the reduction in cardiotoxicity of the doxorubicin was shown. In prepared formulation the PEG and PLGA has a hydrophilic and hydrophobic domain, respectively whereas PEG-PLGA block copolymer may showed amphiphilic properties in an aqueous environment. The prepared block copolymer would be able to self-assemble as a core-carona-type polymeric micelle. In addition, the doxorubicin was incorporated with the F-PEG-PLGA block copolymer and evaluated for their in vitro and in vivo effects.
Material
DOX was obtained as a gift sample from Dabur India Limited, Ghaziabad, India. Folic acid (F), Poly (ethylene glycol)-bis-amine (PEG-bis-amine, Mw:3400), N-hydroxy-succimide (NHS), Dicyclohexylcarbodiimide (DCC), and Poly(D,L-lactide-co-glycolide) (PLGA) (50:50, Mw:8000) were purchased from Sigma-Aldrich. All other reagents including Dichloromethane (DCM), Dimethylsulfoxide (DMSO), Triethylamine (TEA), Diethyl ether were of high performance liquid chromatography (HPLC) grade and were used without further purification.
Animals
The animal studies were conducted with the permission of the Institutional Animal Ethical Committee of Dr. Hari Singh Gour Central University, Sagar, Madhya Pradesh, India. The in vivo study was conducted on albino wistar rats of either sex and 10-12 weeks old (250- 300 g) were used and properly housed.
Method
The overall method for conjugation preparation can be divided into four steps as shown in scheme 1. First step was PLGA activation, second step was conjugation folic acid with PEG bis amine (F- PEG), then preparation of F-PEG-PLGA conjugate in third step and forth step was conjugation of DOX with F-PEG-PLGA to form F-PEG-PLGA-DOX conjugate.14,15
Step I: PLGA activation: PLGA was activated by DCC and NHS in DCM (in molar ratio of PLGA: DCC: NHS=1:1.1:1.1) at room temperature under nitrogen atmosphere for 24 hr. The resultant solution was filtered to remove the by-product dicyclohexylurea (DCU). After filtration, activated PLGA was precipitated by ice-cold diethylether and dried under vacuum.
Step II: Folic acid conjugation with bis amine PEG: The carboxylate group of folic acid was activated by NHS and DCC as described earlier by Yoo and Park16 with a minor modification. In brief, folic acid dissolved in Dimethylsulfoxide (DMSO) and then reacted with NHS and DCC under nitrogen atmosphere at room temperature for 12 h in the molar ratio of 1:2:2.The activated folic acid (0.65 g) was reacted with 0.5 g of NH2-PEG-NH2dissolved in 5 ml of DMSO. The reaction was performed under nitrogen atmosphere at room temperature for 4 h. The resultant solution was diluted with non-solvent for folic acid i.e., acetone and centrifuged. The supernatant was diluted with deionized and dialyzed against deionized water three times (MW cut-off=1000) and freeze-dried.
Step III: Synthesis of F-PEG-PLGA conjugate: The conjugation of activated PLGA with 1.2 equivalents PEG-F was conducted in DMSO at room temperature for 8 h under nitrogen atmosphere. The product was precipitated in ice-cold diethyl ether, dissolved in DMSO for dialysis against deionized water over 48 h (Spectra/Por 7, MWCO: 10,000) and finally freeze dried.
Step IV: Synthesis of F-PEG-PLGA-DOX conjugate: Incorporation of DOX done by the method reported by Yoo and Park15 with little modification. F-PEG-PLGA conjugate dissolved in Methylene chloride and it was activated by adding p-nitro phenyl chloroformate and pyridine (F-PEG-PLGA conjugate: p-nitro phenyl chloroformate: pyridine in stoichiometric molar ratio of 1:2.8:4.8) at 0ºC in nitrogen atmosphere for 3 h. The activated F-PEG-PLGA conjugate dissolved in DMSO and then DOX was added in the presence of TEA for 24 h at room temperature under nitrogen atmosphere (stoichiometric molar ratio of activated F-PLGA-PEG:DOX:TEA=1:1.2:4). Untreated DOX and the other chemicals were removed by dialysis via deionized water, which was followed by three subsequent dialyzing procedures for 4 h (Spectra/Por 7, MWCO: 10,000). The purified F-PEG-PLGA-DOX was freeze-dried and stored at 20ºC for further use.
Synthesis of DOX loaded nano-aggregates: Briefly DOX was neutralized with 2 moles excess TEA in 2 ml of DMSO. The neutralized DOX solution was then added into the 4 ml of DMSO solution with F-PEG-PLGA-DOX conjugates and mixed by vortex for 10 min. The mixture was then transferred for dialysis (Spectra/Por 7, MWCO:10,000) against deionized water for 48 h to produce the nano-aggregates.17
1H-NMR spectrum of conjugates
Nano-aggregates were characterized by 1H-NMR spectroscopy for the confirmation of conjugation F-PEG-PLGA-DOX conjugate formulation. 1H-NMR spectroscopy was carried out using D2O and DMSO as a solvent (Bruker Advanced DRX, 400 MHz, Bruker Optics, Tokyo, Japan). The NMR spectra of each shown as Figure 1.
Morphology study
Transmission Electron Microscope (TEM) analysis was carried out in order to study morphology of the prepared nano aggregates. One drop of the nano-aggregate sample was placed on a 300-mesh copper grid. Excess liquid was blotted with filter paper. The sample was dried in hood for 2 h before examined by a transmission electron microscope (JEM-2010S, JEOL). The TEM photomicrograph of the prepared formulations is shown in Figure 2.
Size, size distribution and surface charge
Average particle sizes of nano-aggregates were measured by the laser light scattering techniques (90-PLUS analyser, Brookhaven Instruments, USA). Samples were prepared by diluting nano-aggregates suspensions with PBS at pH 7.4 and analysed for particle size. Zeta potential of the nano-aggregate was measured by the Laser Doppler Anemometry (Zeta plus, Brookhaven Instruments, USA). The nano-aggregate (~2mg) was suspended in PBS before measurement. Zeta potential measures the surface charge density of the nano-aggregates and it reflects the particle stability in suspension through the electrostatic repulsion between the particles.
Entrapment efficiency
The both nano-aggregate solutions were frozen and lyophilized by a freeze dryer system (Alpha-1-4, Christ, UK) to obtain dried nano-aggregates. The weighted nano-aggregates were dissolved and properly diluted in DMSO. The quantity of DOX loaded in the nano-aggregate was determined by measuring the UV absorbance at 481.0 nm using UV-vis spectrophotometer (Shimadzu 1601, Japan). The entrapped DOX content in the nano-aggregates was calculated from the weight of initial drug loaded nano-aggregate and the quantity of entrapped drug.
Final doxorubicin
Encapsulation efficiency = ------------------------------X 100
Initial doxorubicin
In vitro drug release study
Release of DOX from DN and FDN was evaluated using the dialysis method. Samples were placed in a dialysis bag (MWCO: 2000) and is suspended in a beaker containing phosphate buffer solution (PBS, pH 7.4).The beaker was placed in a shaking water bath and temperature maintained at 37°C. To measure the release of DOX at different time intervals, 1ml of solution was withdrawn from the medium and replaced with 1 ml of fresh PBS to maintain the sink condition. The DOX content in the samples was analysed by using UV-vis spectrophotometry (Shimadzu 1601, Japan) as mentioned above. All experiments were conducted in triplicate.
In vivo blood level and biodistribution studies
Albino Wistar rats (10-12 weeks old, 250-300 g) were chosen for the in-vivo studies. The rats were divided into 4 groups containing six animals in each group. First, second and third groups were treated with plain DOX, DN and FDN formulations, respectively through i.v. route. The dose administered to rat was 6 mg/kg body weight.18 The animals of fourth group did not receive any drug as it served as control group. After administration of the formulation, blood was collected by cardiac puncture method. One rat from each group was sequentially sacrificed and various organs such as heart, lung, liver, kidney and spleen were excised, dried with tissue paper, weighed and homogenized in dichloromethane: isooctane mixture. The extracts were separated by centrifugation at 1200 rpm for 10 minutes. One ml of this was filtered through 0.45-μm-membrane filter and the amount of drug present in each organ was determined by HPLC method as reported previously19 with UV detector at 475 nm. HPLC consist of C-18 column and mobile phase as a mixture of methanol-water [containing 0.1% formic acid anhydrous and 0.1% ammonia solution (25%), pH 3.0], 60:40, with a flow rate of 1.0 mL/min. The column temperature was maintained at 35°C.
Statistical analysis
In order to evaluate the extent of a relationship between two data sets, Pearson’s correlation coefficients were used. To analyse statistical differences among groups one-way analysis of variance was used. A p value of 0.05 was considered statistically significant.
Nano-aggregates bearing DOX was synthesized as shown in scheme 1. The biodegradable block copolymer PLGA-PEG with folic acid was synthesized by the previously reported methods14-17,20,21 with slight modification. The folic acid was used in the formulation to make tumor specific system, but in present paper the author try to describe a partial part of study i.e. reduction of cardiotoxicity of DOX after conjugation. DOX-loaded nano-aggregates were prepared with the help of amphiphilic block copolymers which form a nano-aggregate structure through the association of the hydrophobic segments in a hydrophilic solvent. In this research paper we hypothesized that DOX incorporated nano-aggregates system was prepared which was decorated with folic acid (F-PEG-PLGA-DOX). The prepared system may serve as a better drug delivery approach for tumor targeting with reduce cardiotoxicity of DOX due to the over expression of folic acid receptors on cancerous cells.
In the first part of synthesis the PLGA and folic acid was activated, separately with the help of DCC & NHS. In the next step folic acid PEG conjugates was formed by using bis amine PEG with activated folic acid in presence of DMSO. The folic acid was conjugated with the -NH2terminal of bis amine PEG by formation of amide linkage between the -COOH group of folic acid and -NH2group of PEG. In subsequent step the activated PLGA conjugated with F-PEG in presence of DMSO at room temperature under nitrogen atmosphere. Then DOX conjugation was performed by using previously reported method15 with slight modification. The F-PEG-PLGA was conjugated with DOX in presence of TEA under nitrogen atmosphere. The -NH2 group of PEG conjugate with –COOH terminal of DOX. The NMR study was performed to confirm the formation of F-PEG-PLGA-DOX conjugates which is shown in Figure 1 (Scheme 1).
The NMR spectrum demonstrates various peaks related to F-PEG-PLGA-DOX polymer. The protons associated with folate were indicated by the small peaks at 6.6, 7.6 and 8.7 ppm. The -CH3and-CH peaks of PLA block was obtained at 1.5 and 5.2 ppm, respectively while the peak at 4.8 ppm due to -CH2-proton of PGA. The spectrum also shows a peak at 3.5 ppm which may be of a characteristic peak of PEG. The corresponding peaks of folate was observed as a small peaks at 7.1 ppm, 8.1 ppm and 1.2 ppm, while the corresponding peaks of DOX was obtained as the peak at 1.2 ppm and 4 ppm originated from -CH3 proton, 7.6 ppm and 8.1 ppm originated from -CH proton, 2.8 ppm and 3 ppm originated from -CH2-proton and peak of 4.5 ppm originated from -OH proton of doxorubicin. Hence, the NMR spectrum confirms the formation of F-PEG-PLGA-DOX conjugate. The prepared non-aggregates were characterized for various parameters like surface morphology, aggregates size, size distribution, surface charge, % entrapment efficiency, and in-vitro drug release studies. The biodistribution studies of both the formulation were also performed to evaluate the effect of DOX conjugation (with F-PEG-PLGA) on the cardiotoxicity of the drug.
The morphology of nanoscopic nano-aggregates was measured by transmission electron microscopy (TEM). TEM analysis of nano-aggregates formulations showed that the prepared aggregates were spherical and relatively uniform in size as shown in Figure 2. The size range of most of the nano-aggregates was lies in between 100-170 nm. Average aggregate size was measured by laser scattering technique (90-plus analyser, Brookhaven Instrument). The aggregate size for formulations, FDN and DN were found to be 141±0.8 nm and 135±0.6 nm, respectively as shown in Table 1. Zeta potential of nano-aggregates preparation was measured by laser Doppler anemometry (Zeta plus, Brookhaven Instruments). The DN formulation shows the positive zeta potential value attributed due to the presence of amine group of bis PEG and negative zeta potential value FDN is due to the presence of anionic carboxylic groups at the terminals point in the polymer and values of zeta potential were shown in Table 1.
S. No. |
Formulations |
Particle Size (nm) |
Entrapment efficiency (%) |
Zeta Potential (mV) |
1 |
DOX bearing nano-aggregates (DN) |
135±0.6 nm |
79.3±0.5% |
4.8 |
2 |
Folic acid conjugated DOX bearing nano-aggregates (FDN) |
141±0.8 nm |
71.7±0.5% |
-2.3 |
Table 1 Particle size, Percentage entrapment efficiency and Zeta Potential of nano-aggregates
The % entrapment efficiency of the DN and FDN was determined by dialysis method. The drug entrapment efficiency of DOX nano-aggregates DN and FDN were found to be 79.3±0.5% and 71.7±0.5%, respectively as shown in Table 1. The higher % entrapment efficiency may be attributed to lipophilic nature of DOX which helps in entrapment of drug in the lipophilic inner core by covalent binding. Presence of PEG may be one of the reasons for higher drug entrapment.22
The in vitro drug release study was performed by using PBS (pH 7.4). The results of in-vivo studies showed that DOX release rate was faster in first 10 h and then it reached to plateau condition within 24 h. The initial higher release pattern may be due to initial burst release of DOX from formulation. Further release pattern confirm the sustain release of drug from the formulation for longer period of time.
The in-vivo biodistribution studies were performed by using albino wistar rats. The plain DOX, DN and FDN were administered I.V. with the dose of DOX at 6 mg/kg body weight. The organ distributions of different formulations were shown in Figure 5, 6. In case of plain DOX, the maximum concentration of drug was found to be in heart (i.e. approximately 12.5%) and in liver 13.8 % after six h. After 24 h, the DOX concentration were found to be decreased and it was 9.3 & 8.1 in heart & liver, respectively. The higher uptake of DOX is responsible for the cardiotoxicity of the drug. The blood profile studies showed that initial concentration of DOX was found to be 15.2% after 6 h whereas after 24 h it was decreased to 2.3%. These values indicate that the drug going to be either absorbed as well as excreted from the body. The higher percentage of plain DOX in lung and spleen as well as liver indicates the RES uptake of the drug. The percentage of drug in kidney was found to be 5.2 and 0.8 after 6 and 24 h, respectively. These results indicated that most of the DOX eliminated from the body within 24 h. In case of DN the percentage heart uptake was found to be 5.6 in 6 h and 2.1 after 24 h respectively. Whereas in case of FND the concentration of DOX was found to be 4.3 and 1.2 % after 6 and 24 h, respectively. The results of heart uptake studies showed the approximately 2.5 times and 2 times less absorption of DOX in case of FDN and DN, respectively as compare to plain DOX. The less uptake of DOX in prepared FDN and DN may be due to less absorption of polymeric conjugates by the heart muscles which leads less cardiotoxicity of DOX. Although the mechanism accountable for reduction in DOX cardiotoxicity yet to be clear. As compare to plain DOX, the concentration of DOX was found to be very less in liver, lung, spleen in case of FDN and DN formulations. This may be due to less recognisation of drug by RES organs due to stearic hindrance because of the presence of PEG. The blood profile studies indicate that the FDN and DN formulations were available in the blood for longer period of time as compared to free DOX. The concentration of DOX was found to be approximately 3.0 to 3.5 and 1.5 times higher in 6 h and 24 h, respectively in case of both the nano-aggregates formulations i.e. FDN and DN. This blood profile studies indicates that the DOX in nano-aggregates formulation will be available for the absorption at target site for a longer period. Ultimately these nano-aggregates formulations may enhance the tumor uptake of DOX with reduced cardiotoxicity. The presence of folic acid as the surface modifier makes these nano-aggregates as target specific system to the cancerous cells due to the presence of folic acid receptors at the surface of the cancerous cell. Folic acid conjugated nano-aggregates will bind to the cancerous cell and shows sustain release of drug. Hence more of the drug will be available at the target cell which will be discussed with further communication as the studies are in progress.
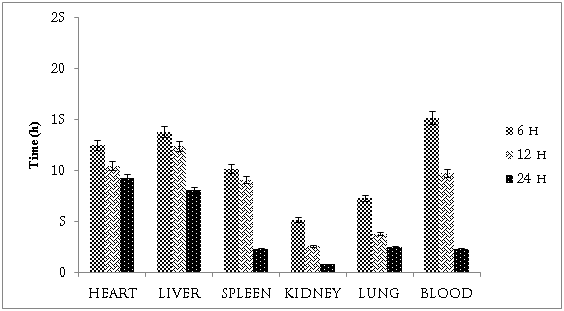
Figure 4 Distribution of DOX in various organs of albino rats after i.v. administration of plain doxorubicin.
S.D.± Mean (n=3)
The prepared nano-aggregates were evaluated for the effect on cardiotoxicity of the DOX after copolymers conjugation. Due to hydrophilicity of DN and FDN, a significant difference in biodistribution characteristics of the DOX was observed. The biodistribution results showed the cardiac uptake was higher in free DOX as compared to the FDN and DN Nano-aggregates formulations. Hence, it can be concluded that the prepared nano-aggregates formulations may reduce cardiotoxicity of DOX and may available for the absorption for a longer period of time to the target organ.
Authors acknowledge UGC India, for financial support. We thank Dabur India Limited, Ghaziabad, India for providing Doxorubicin as a gift sample. Authors extend their acknowledgement to Punjab University, Chandigarh, India, for providing the TEM facility. The author expresses his sincere thanks to Banaras Hindu University (Varanasi, India) for providing IR facility.
Author declares that there is no conflict of interest.

©2016 Soni, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.