eISSN: 2379-6367


Research Article Volume 4 Issue 6
1Department of Pharmaceutical Health Outcomes and Policy, University of Houston, USA
2Scientist, Department of Health Economics and Outcomes Research, Extro Pharm, China
3Professor, Department of Pharmaceutical Health Outcomes and Policy, University of Houston, USA
Correspondence: Sujit S Sansgiry, Department of Pharmaceutical Health Outcomes and Policy, University of Houston, Texas, USA , Tel 8328155452
Received: August 20, 2016 | Published: November 14, 2016
Citation: Huei YL, Gupta P, Sawant R, et al. A cost-effectiveness comparison of alosetron, eluxadoline, and rifaximin in the treatment of irritable bowel syndrome with diarrhea. Pharm Pharmacol Int J. 2016;4(6):450-458. DOI: 10.15406/ppij.2016.04.00095
Objective: The recurrent and complex Irritable Bowel Syndrome (IBS) has major impact on healthcare cost and quality-of-life (QoL) with highest prevalence and lowest QoL score of diarrhea predominant IBS (IBS-D). However, to treat IBS-D, Alosetron, Eluxadoline and Rifaximin are the only FDA-approved medications. There is a lack of standardized guidelines to recommend optimal therapy. Therefore, the aim of the study was to develop a decision-making analytical model to evaluate the economic and humanistic implications of the three approved medications in individuals with IBS-D.
Method: This study included estimates from clinical trials which measured IBS quality of life (IBS-QoL) for each treatment compared to placebo. The costs for individual treatment arm were the average wholesale prices from Red Book. A 12week cost-effective decision-making analytical model was developed. The costs for the associated adverse events were weighted by the respective probabilities to evaluate the cost per quality-adjusted life year (QALY) gain and incremental cost-effectiveness ratio (ICER) as the study outcomes.
Results: The management cost for unit increment of QALY with respect to placebo was the least for Rifaximin treatment ($21,457); and the highest for alosetron treatment ($138,111). The ICER values of base case indicated dominance of Rifaximin over Eluxadoline by $291,123; and of Eluxadoline over alosetron by $482,767. In a willingness-to-pay range of $50,000 to $100,000, Rifaximin is the preferred option for 80% of IBS-D patients.
Conclusion: The results indicate that Rifaximin was the most cost-effective treatment among all the three regimens to manage IBS-D symptoms based on the parameters considered in this study.
Keywords: irritable bowel syndrome, alosetron, rifaximin, eluxadoline, pharmacoeconomics, cost-effectiveness
CEA, cost-effective analysis; IBS, irritable bowel syndrome; IBS-D, irritable bowel syndrome diarrhea; IBS-QoL, ibs quality of life; HRQoL, health related quality of life; ICER, incremental cost effectiveness ratio; WTP, willingness to pay; NMB, net monetary benefit
Irritable Bowel Syndrome (IBS) was first defined in 1978, and is characterized by abnormal pain, bloating, discomfort and disturbed bowel habits. IBS is a chronic and relapsing gastrointestinal disorder with global prevalence of 5-20%.1-3 This disorder consists of four subtypes: IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), mixed bowel pattern (IBS-M), and unclassified IBS.1,2 Options for IBS treatment include changes of diet, antispasmodics, exercise, laxatives, anti-diarrheals, antidepressants and serotonin agents.4 Although a variety of pharmacological agents have been provided to manage IBS symptoms, there are still no conclusive treatment strategies for this disorder.
Due to the frequency of symptoms and lack of curative agents for IBS, the disease imposes huge economic and health burden on the patient. As per a recent report published on May 2016 by International foundation for gastrointestinal disorder, in US alone there are between 2.4-3.5 million annual physician visits with estimated direct and indirect costs of $21 billion or more.5 Chronic and recurrent IBS costs the patient both financially and from the humanistic perspectives: cost of prescription medications, over-the-counter treatments, emergency-room visits, and hospitalizations; and cost of lost productivity, and decreased quality of life (QoL). A systematic review regarding the annual economic burden of IBS in the United States, attributed 1.35 billion dollars and 200 million dollars to direct and in direct costs associated with IBS, respectively.6 Furthermore, on average, patients with severe refractory IBS symptom suffer for24 days per month with abdominal pain, and for 145 days in a year their activities were restricted by these symptoms.7 Considering IBS symptoms consumes a huge amount of money in medications, incurs large effect on the productivity of the patient, and impairs QoL among IBS patients; more effective medications in treating multiple symptoms of IBS may be urgently needed.
Of all the IBS subcategories, IBS-D affects up to 40% of the IBS patients.8 These IBS-D patients also have the lowest score of QoL compared to other IBS patients because of the significant influence of diarrhea on their social activities and daily life.9 Despite that in the year 2000, alosetron, a5HT3 receptor antagonist, was the only approved medication for the condition and that too solely for women with severe diarrhea.10 In 2015, two additional drugs were approved to treat IBS-D for the US market: Eluxadoline and Rifaximin. Eluxadoline, known as a mu- and kappa-opioid receptor agonist, and a delta-opioid receptor antagonist, was proven safe and effective in two phases of three clinical trials comparing placebo over 24 weeks.11 Rifaximin is an antibiotic derived from Rifampicin, that manages the overgrowth of intestinal bacteria, a pathogenesis of IBS-D.12 Despite much evidence of efficacy and safety for both Eluxadoline and Rifaximin for IBS-D from various phase II and III clinical trials, there is no standard treatment or guideline to follow for patients with IBS. In addition to that, the high costs of medications and of potential adverse events create a much more complex situation to decide which medication may be the best for IBS-D patients. Therefore, the aim of the study was to develop a decision-making analysis model to evaluate the economic and humanistic implications of the three aforementioned approved medications, i.e. Alosetron, Eluxadoline, and Rifaximin, in individuals with IBS-D. The hypothesis of this study is - Rifaximin is the most optimal treatment to manage IBS-D because of its relatively short treatment period, minor side effects, and lengthy effectiveness time of up to 12 weeks.
This study provides comparative information on economics and effectiveness for three medications, and thus may serve as a good decision-making tool for further IBS-D management. This is the first study that examines and compares the clinical and economic outcomes of these three FDA-approved medications to manage IBS-D for the United States that we are aware. Additionally, the humanistic implications of these outcomes make this study a good reference for physicians to consider while making prescribing decisions for patients with IBD-D.
Study design
In order to develop a cost-effectiveness decision analytical model for the three approved medications for IBS-D patients, this study used the clinical data from the existing randomized controlled trials (RCTs) and cost data from Red Book. Study design of this study was from patient’s perspective. The decision analysis was developed by following the effectiveness and adverse events of the treatment over a 3 month medication treatment cycle similar to the trials assuming the cycle length to be sufficient for IBS-D management. Parameters of utilities and probabilities of events were obtained from clinical trials as well (Table 1) (Table 2).
Sample selection
From different clinical trials of alosetron, Rifaximin, or Eluxadoline potential diarrhea-predominant IBS patients were identified using Rome's criteria. Clinical trials that had reported health-related quality-of-life (HRQoL) were included in this study. The included trials were: Target 1 and Target 2 for Rifaximin12,13 SBA3001 and S3BA3002 for alosetron14 and IBS-3001 and IBS-3002 for Eluxadoline.11
Data Collection Methods
Clinical data
The cycle length of each treatment considered was 12weeks.Three medication regimens were considered as: alosetron 1 mg twice daily for 12 weeks Eluxadoline 100 mg twice daily for 12 weeks and Rifaximin 550 mg three-times daily for 2 weeks. All values of HRQoL were measured at the time of starting the interventions. The values of IBS-adequate relief were assumed to represent the utilities of the treatments because of its positive effect on treatment continuation. Regarding the safety of treatments, only the adverse events with ³5% probability were included in the decision making analysis (Table 1).
Cost data
The average wholesale prices (AWP) of medications were derived from the 2016 Red Book.15 For example, AWP of Rifaximin 500 mg is $9.2 per unit. For medications with more than one AWP, the average price was used. For example, 1 mg alosetron has two AWPs: $52.05 and $58.90; therefore, the average AWP of 1 mg alosetron: $55.47 was used to calculate the cost of a regimen. Other costs, such as cost of adverse events, were converted into 2016 US dollars using 3% annual inflation rate (Table 1). For example, the cost to manage constipation was USD 859.8 in 2010; after converting to the US 2016 dollars, it costs $1,032.1.
Outcomes
The main effectiveness outcome for this study was HRQoL. It was assessed in terms of the cost per unit quality-adjusted life years (QALY) gained. In the decision analytic model, the increment cost-effectiveness ratio (ICER) was calculated for comparative assessment for various treatment arms.
Data analysis
The HRQoL associated with IBS symptoms was accurately estimated using a disease-specific question naire, Irritable Bowel Syndrome Quality-of-Life (IBS-QoL). However, there was more than one version for IBS-QoL. For example, in 1998 Patrick et al. provided 34items in his IBS-QoL questionnaire, covering Dysphoria, activity interference, body image, health worry, food avoidance, social reaction, and sexual relationship's domains.16-18 Hahn et al. in 1997, on the other hand, believed that a 30-item questionnaire, including emotional health, mental health, sleep, energy, physical functioning, diet, social role, physical role, and sexual relations domains, was more accurate to measure the HRQoL of IBS.19,20 Both the versions of IBS-QoL questionnaire measure items on a 0-100point scale whereby 0 represents death, and 100 represent perfect health.
Unfortunately, not all the patients of these three medications were measured with same IBS-QoL questionnaire patients on Rifaximin and Eluxadoline were measured with Patrick’s 34-item questionnaire and patients using alosetron were measured by Hahn’s 30-item questionnaire.19,20 In order to cope with varied scales of IBS-QoL, indirect comparisons of HRQoL were made using the difference in IBS-QoL estimates between placebo and medication groups for these three medications. To eliminate the potential bias caused by different baselines in clinical trials known as the placebo effect, we considered the differences between the treatment and placebo as the effectiveness measure. The cost outcome, cost/QALY gained, was the additional cost incurred for unit increment in QALY (i.e. for 3-month cycle, 100% increment in IBS-QoL score or HRQoL). For this measurement, the difference in costs between the medication and the placebo group was calculated and was divided by increased percentage of IBS-QoL score across the two groups. The costs' values included the cost(s) of medication(s) and of managing adverse events in monetary terms.
For a cost-effective treatment, the recommended willingness-to-pay (WTP) to manage IBS-D was considered in the USD of 50,000-100,000/QALY.21-24 Using decision-analytic model net change in IBS-score and associated costs was obtained for three treatment arms. The 2 estimates were used to calculate ICER values of alosetron with respect to Eluxadoline and of Eluxadoline with respect to Rifaximin as the reference arm. The costs and probability estimates for each event were presented in Table 1 and the decision analytic model in Figure 1. The values of costs as entered in this model were weighted by the probability of the event. Sensitivity analysis was conducted for various parameters using the range of published values to take into account the uncertainty in the parameter estimations. The robustness of the model was analyzed through one-way sensitivity analysis. The minimal and maximal values for costs and probabilities of different events were obtained from literature and can be referred from Table 1.15,26-28 The statistical analysis of the decision-model was performed with the Tree Age Pro 2009 Health Care program and Microsoft Excel 2010.25
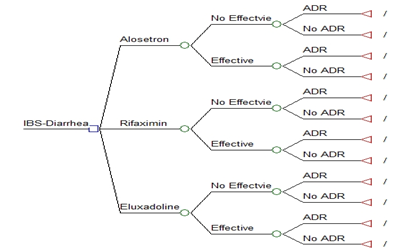
Figure 1 The decision model of diarrhea-predominant irritable bowel syndrome in 12-week interval
Square: decision node; circle: chance node; triangle: terminal node; ADR: Adverse Events.
Alosetron 1 mg twice daily for 12 weeks; Eluxadoline 100 mg twice daily for 12 weeks; and Rifaximin 550 mg three-times daily for 2 weeks. The first decision node is effectiveness of the treatment, and followed by a node for adverse events. The adverse events are enlisted in the model in order of decreasing probability of occurrence. The adverse events with probability ³ 0.5% only were included in the decision model.
Components |
Costs [USD] |
Year |
Probability (SD) |
Cost (Range) [2016 USD] |
Reference |
|
Alosetron |
0.570 (0.290-0.710) |
9,319.50 |
(9,300.9-9841.4) |
|||
Constipation |
444 |
2003 |
0.290 (0.095-0.382) |
659.7 |
(606.2-744.4) |
|
Abdominal Pain |
2690.5 |
2011 |
0.070 (0.071-0.185) |
3,133.10 |
(1,370.6-6,212.6) |
|
Nausea |
2494.3 |
2011 |
0.060 (0.034-0.090) |
2,904.60 |
(1,286.5-5,775.7) |
|
Rifaximin (Target 1 & Target 2) |
0.410 (0.400-0.420) |
386.4 |
(289.8-386.4) |
|||
Headache |
489 |
2003 |
0.061 (0.050-0.100) |
726.6 |
(660.0-797.9) |
|
Upper respiratory tract infection |
3745.3 |
1984 |
0.056 (0.013-0.060) |
9,926.30 |
(8,410.9-10,029.0) |
|
Eluxadoline (IBS3001& IBS3002) |
0.654 (0.529-0.610) |
3,225.60 |
(2,822.4-6,451.2) |
|||
Constipation |
444 |
2003 |
0.080 (0.074-0.086) |
659.7 |
(606.2-744.4) |
|
Nausea |
2494.3 |
2011 |
0.077 (0.075-0.081) |
2,904.60 |
(1,286.5-5,775.7) |
|
Abdominal Pain |
2690.5 |
2011 |
0.065 (0.058-0.072) |
3,133.10 |
(1,370.6-6,212.6) |
|
Table 1 Components of total costs for treatment in 3-month period in 2016 US dollar
Note:
The annual inflation rate of cost is 3%; and SD is 95% standard deviation.
The treatment groups: Alosetron represents alosetron 1 mg twice daily for 12 weeks; Rifaximin represents rifaximin 500 mg three-times daily for 14 days; and Eluxadoline represents eluxadoline100 mg twice daily for 12 weeks.
USD: in United States dollar.
Base-case analysis
The Table 2 represents the comparative gain of HRQoL and the associated cost for each treatment group with respect to the placebo group. For each of these groups, compared to placebo, the HRQoL gain was 7.2% for alosetron, 4.6% for Rifaximin, and 5.0% for Eluxadoline; which yielded the cost estimates of $9,944, $987, and $3,706, respectively. Therefore, the associated cost for unit increment of QALY with respect to placebo for Rifaximin treatment was $21,457; for Eluxadoline treatment was $74,120 and for alosetron treatment was $138,111, respectively. In decision analysis (Table 3) (Figure 2) net percentage changes of IBS-QoL score were: for Rifaximin 1.89; for Eluxadoline 2.82; and for alosetron 4.10, respectively. The ratios of cost per IBS-QoL (%) from the decision model were $52,311 for Rifaximin; $131,407 for Eluxadoline; and $241,335 for Alosetron, respectively. The incremental cost-effectiveness ratio (ICER) of the base case for Eluxadoline was $291,123 compared with Rifaximin and for Alosetron was $482,767 compared with Eluxadoline, respectively.
Medication |
Placebo |
Intervention |
HRQoL (%) |
Costs ($) |
Cost/QALY ($) |
Reference |
||
Mean |
CFB |
Mean |
CFB |
|||||
Alosetron 1 mg |
60.89 |
14.7 |
67.48 |
21.9 |
7.2 |
9,944 |
138,111 |
|
Rifaximin 550 mg |
48.3 |
15.8 |
61.3 |
20.4 |
4.6 |
987 |
21,457 |
|
Eluxadoline 100 mg |
63.9 |
17.8 |
70 |
22.8 |
5 |
3,706 |
74,120 |
|
Table 2 Estimated Health related quality of life (HRQoL) and the associated cost using Irritable Bowel Syndrome Quality-of-Life (IBS-QoL) Questionnaire
Note:
CFB: Change in IBS-QoL score from baseline; HRQoL: Health-related quality-of-life calculated as difference in IBS-QoL score between intervention and placebo (range: 0-100, 0 means dead and 100 means perfectly healthy); QALY: Quality-adjusted life years calculated for 100% change in HRQoL over a 3-month cycle.
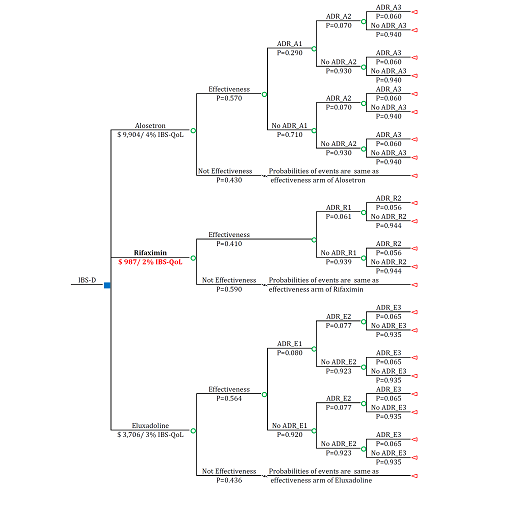
Figure 2 The outcome of the decision model of diarrhea-predominant irritable bowel syndrome in 12-week interval
Blue square: Decision Node; Green Circle: Chance Node; Triangle: Terminal Node; ADR: Adverse Events; No ADR_: No Adverse Event; IBS-D: Irritable Bowel Syndrome–Diarrhea; ADR_A1=ADR_E1: Constipation; ADR_A2=ADR_E3: Abdominal Pain; ADR_A3=ADR_E2: Nausea; ADR_R1: Headache; ADR_R2: Upper Respiratory Tract Infection; P: Probability of the Clinical Event.
Alosetron increases 4% IBS-QoL which cost $9,904; rifaximin increases 2% IBS-QoL which cost $987; and eluxadoline increases 3% IBS-QoL which cost $3,706. Among all treatments, rifaximin is considered as the optimal treatment in willingness-to-pay range of $50,000-$100,000.
In order to test the robustness of the decision model, a Monte Carlo simulation test was also performed with 10,000 samples; outputs were presented in Table 3. Net percentages of IBS-QoL from simulation were: for rifaximin1.79, for Eluxadoline 2.85 and for alosetron 4.13, respectively. The ratios of cost per IBS-QoL (%) from the decision model were: $52,517 for Rifaximin; $131,103 for Eluxadoline and $241,303 for alosetron, respectively. The ICER values as obtained from Monte Carlo simulation were: $283,707 for Eluxadoline compared with Rifaximin and $483,878 for alosetron compared with Eluxadoline, respectively.
Base case |
||||||
|
Total costs |
Net IBS-QoL (%) |
Cost ($)/ IBS-QoL (%) |
Incremental IBS-QoL (%) |
Incremental Costs |
ICER |
Rifaximin |
$987 |
1.886 |
$52,311 |
_ |
_ |
_ |
Eluxadoline |
$3,706 |
2.82 |
$131,407 |
0.933 |
$2,719 |
$291,123 |
Alosetron |
$9,904 |
4.104 |
$241,335 |
1.284 |
$6,199 |
$482,767 |
Monte Carlo simulation |
||||||
Rifaximin |
$978 |
1.789 |
$52,517 |
_ |
_ |
_ |
Eluxadoline |
$3,698 |
2.85 |
$131,103 |
1.061 |
$2,721 |
$283,707 |
Alosetron |
$9,900 |
4.133 |
$241,303 |
1.283 |
$6,201 |
$483,878 |
Table 3 Incremental results of base case and Monte Carlo simulation for 12-week treatment
Note:
IBS-QoL: 0-100% (0 means dead and 100% means perfectly healthy); Net IBS-QoL: (change of placebo IBS-QoL from treatment initiation) – (change of treatment IBS-QoL from treatment initiation); ICER: incremental cost-effectiveness ratio.
The cost-effectiveness analysis diagram for the base-case, as presented in Figure 3, indicates Rifaximin, Eluxadoline and alosetron were roughly in a line of low cost to high cost, and of low effectiveness to high effectiveness, i.e. in the northeast quadrant of the cost-effectiveness plane, thereby indicating the dominance of Rifaximin among the three treatment options. In addition, the acceptability curve from the Monte-Carlo simulation (Figure 4) indicates that for the treatment management cost of up to $40,000, all the patients would be willing to pay for Rifaximin. However, at a cost of $45,000 and more, the probability to use Rifaximin was 0.8, and to use Eluxadoline was 0.2, respectively.
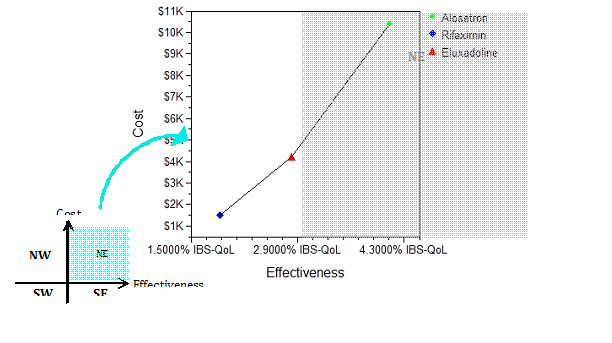
Figure 3 Cost-effectiveness of IBS with diarrhea
IBS: Irritable Bowel Syndrome; IBS-QoL: IBS-related quality of life, range from 0-100.
Compared to placebo, rifaximin increases by 1.89% IBS-QoL with $1,000 cost; eluxadoline increases by 2.82% IBS-QoL with $4,000 cost; and alosetron increases by 4.10% IBS-QoL with $10,000 cost.
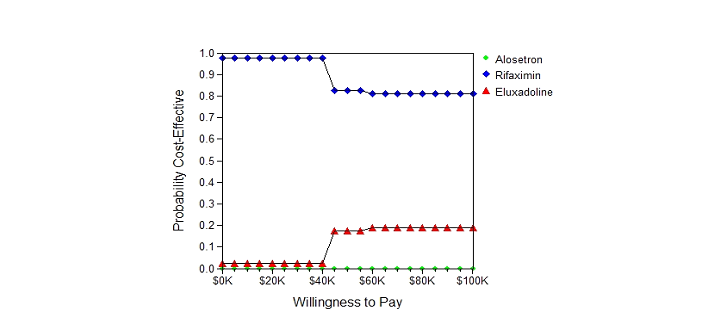
Figure 4 Acceptability curve using Monte Carlo simulation (10,000 times)
When the willingness-to-pay (WTP) is under or equal to $40,000, rifaximin is the only medication to use to manage Irritable Bowel Syndrome-diarrhea (IBS-diarrhea). For a WTP of $45,000 or more, 80% of IBS-diarrhea patients will use rifaximin, and 20% of IBS-diarrhea patients will use eluxadoline to manage IBS symptom.
Sensitivity analyses
Outcomes of one-way sensitivity analysis for individual variables were similar where alosetron treatment costs was highest with maximum IBS-QoL improvement and Rifaximin treatment costs was least with minimum IBS-QoL. In addition, since Rifaximin was the most cost-effective treatment to manage diarrhea predominant IBS symptom. The Tornado Diagram analysis (Figure 5) provides the net monetary benefit (NMB) changes for variables associated with Rifaximin treatment at a WTP threshold of $50,000: cost of Rifaximin, costs of adverse events of Rifaximin, and the probabilities of adverse events of Rifaximin. Among those variables, the probability of upper respiratory tract infection attributes the widest NMB of $478.5 and the cost of headache has the least NMB of $8.4.
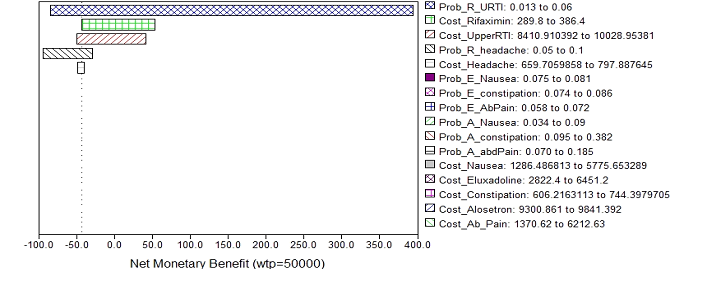
Figure 5 Tornado diagram of sensitivity analysis
WTP: Willingness To Pay; Prob_R_URTI: Probability Of Upper Respiratory Tract Infection When Using Rifaximin; Cost_Rifaximin: Cost Of Rifaximin; Cost_Upperrti: Cost To Manage Upper Respiratory Tract Infection; Prob_R_Headache: Probability Of Headache When Using Rifaximin; Cost_Headache: Cost To Manage Headache; Prob_E_Nausea: Probability Of Nausea When Using Eluxadoline; Prob_E_Constipation: Probability Of Constipation When Using Eluxadoline; Prob_E_Abdpain: Probability Of Abdominal Pain When Using Eluxadoline; Prob_A_Nausea: Probability Of Nausea When Using Alosetron; Prob_A_Constipation: Probability Of Constipation When Using Alosetron; Prob_A_Abdpain: Probability Of Abdominal Pain When Using Alosetron; Cost_Nausea: Cost Of Nausea; Cost_Eluxadoline: Cost Of Eluxadoline; Cost_Alosetron: Cost Of Alosetron; Cost_Ab_Pain: Cost To Manage Abdominal Pain.
When willingness to pay is $50,000, rifaximin is the dominant treatment to manage Irritable Bowel Syndrome-diarrhea. Among all variables associated with rifaximin, the uncertainty of probability of upper respiratory tract infection is 91.33%, of rifaximin cost is 3.72%, of the cost to manage upper respiratory tract infection is 3.26%, of the probability of headache is 1.6%, and of the cost to manage headache is 0.03%.
Although, there were only three medications approved by the FDA for diarrhea predominant IBS patients, the high cost of treatment and the huge impact on quality life of this recurrent condition makes the choice for managing IBS-D quite difficult yet important. Considering the cost for IBS-D management to gain one QALY, Rifaximin costs the least amount of money of all the treatments. In addition, both base case decision analysis and Monte Carlo simulation indicate that for the majority of IBS-D patients, in a willingness-to-pay range of $50,000 to $100,000, Rifaximin was the most cost-effective treatment. All these observations validate the hypothesis of this study, which recommends prescribing Rifaximin as the most optimal option to manage IBS-D symptoms.
Even though the WTP range was for a budget of one year, and the cycle length was for 3-months only, the study results were still trustworthy for the following reasons:
Another interesting observation of this study was the fact that alosetron is the least preferred therapy despite showing highest improvement in HRQoL (Figure 4). In the year 2003, a study published by Ladabaumet al. estimated $358,700 as the cost of gaining one QALY for a 45-year female IBS-D patient using alosetron. This estimated cost was for the year 2000, and it calculated QALY using adequate relief.21 Contrary to this, our study estimated QALY by calculating HRQoL estimates from IBS-QoL questionnaire for a 3 month cycle. Our study found that in the year 2016, it costs $138,111 to treat the same patient. Despite a difference in the evaluation method of QALY and the associated costs in both the studies, their results suggest that alosetron is an effective treatment in managing IBS symptom and for improving patient’s HRQoL. However, a high cost of alosetron decreases its accessibility for all IBS-D patients. A comprehensive comparison of three FDA approved treatment options for IBS-D and not just alosetron, as in Ladabaum’s study, makes our study a better source of reference for making a decision from the patient’s perspective.
Although this study was innovative, there are some limitations of this study. First, the questionnaires utilized to measure IBS-QoL among three treatments were different, and thus, the values of IBS-QoL might not be directly comparable. There was no standard IBS questionnaire to evaluate quality of life of diarrhea predominant IBS patients, resulting in evaluations of IBS-D that might measure slightly different domains from each questionnaire. Therefore, a change in IBS-QoL questionnaire might affect the results. In fact, for future studies, this could be an important step towards validating the findings of this study.
Second, the severities of diarrhea of IBS in clinical trials probably were not the same among three treatments. For example, the quality of life in Rifaximin and Eluxadoline clinical trials was measured using the same 34- item questionnaire, but in a 3-month cycle, the IBS-QoL scores of placebo groups in these two clinical trials were different: 48.3% of Rifaximin clinical trial, and 63.9% of Eluxadoline clinical trial. Even the change in IBS-QoL from baseline showed varied results. The difference between placebo groups in different clinical trials indicates a high possibility of different stages of severity of IBS with diarrhea.
Finally, this study simulates only 3-months IBS-D in a year which might not be realistic because the IBS-D is a recurrent symptom, and it can be replaced by other subtypes. This study could not include all the possible symptoms into consideration because the associated clinical information was still not available. Lack of including the real-world data, was a major limitation of this study. This could possibly be the reason for observing low variation in 10,000 Monte-Carlo simulations. For future studies, it is necessary to have long-term real-world follow-ups with IBS patients and develop a more realistic model to include diarrhea, constipation, mixed and non-specified IBS.
In conclusion, this study provides us evidence-supported information regarding the costs and effectiveness among three potential treatments for IBS-D patients. The least treatment management costs with adequate effectiveness support Rifaximin to be the best option among the three treatments. On the other hand, despite the highest effectiveness of alosetron, the highest associated treatment management cost makes alosetron a therapy with lowest accessibility compared to other medications. Future studies, need to include long-term real-world data to validate these findings.
None.
Author declares that there is no conflict of interest.

©2016 Huei, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.