In this paper, compact effective potentials, charge distribution, Atomic Polar Tensor (APT) and Mulliken charges were studied using a unrestricted Hartree-Fock computational simulation in a protonated rhodochrosite crystal. The rhodochrosite crystal unit cell of structure CMn6O8, where the charge distribution by the molecule was verified in the UHF CEP-4G (Effective core potential (ECP) minimal basis), UHF CEP-31G (ECP split valance) and UHF CEP-121G (ECP triple-split basis). The largest load variation in the APT and Mulliken methods were obtained in the CEP-121G basis set, with δ=2.922 e δ=2.650 u.a., respectively, being δAPT> δMulliken. The maximum absorbance peaks in the CEP-4G, CEP-31G and CEP-121G basis set are present at the frequencies 2172.23 cm-1, with a normalized intensity of 0.65; 2231.4 cm-1 and 0.454; and 2177.24 cm-1 and 1.0, respectively. An in-depth study is necessary to verify the absorption by the tumoral and non-tumoral tissues of rhodochrosite, before and after irradiating of synchrotron radiation using Small–Angle X–Ray Scattering (SAXS), Ultra–Small Angle X–Ray Scattering (USAXS), Fluctuation X–Ray Scattering (FXS), Wide–Angle X–Ray Scattering (WAXS), Grazing–Incidence Small–Angle X–Ray Scattering (GISAXS), Grazing–Incidence Wide–Angle X–Ray Scattering (GIWAXS), Small–Angle Neutron Scattering (SANS), Grazing–Incidence Small–Angle Neutron Scattering (GISANS), X–Ray Diffraction (XRD), Powder X–Ray Diffraction (PXRD), Wide–Angle X–Ray Diffraction (WAXD), Grazing– Incidence X–Ray Diffraction (GIXD) and Energy–Dispersive X–Ray Diffraction (EDXRD). Later studies could check the advantages and disadvantages of rhodochrosite in the treatment of cancer through synchrotron radiation, such as one oscillator crystal.
Keywords: rhodochrosite, quartz crystal, hartree-fock methods, apt, mulliken, effective core potential, synchrotron radiation, cancer, tumoral tissues
Hartree-Fock methods
The Hartree-Fock self–consistent method14–20 is based on the one-electron approximation in which the motion of each electron in the effective field of all the other electrons is governed by a one-particle Schrodinger¨ equation. The Hartree-Fock approximation takes into account of the correlation arising due to the electrons of the same spin, however, the motion of the electrons of the opposite spin remains uncorrelated in this approximation. The methods beyond self-consistent field methods, which treat the phenomenon associated with the many-electron system properly, are known as the electron correlation methods. The vast literature associated with these methods suggests that the following is a plausible hierarchy:
The extremes of ‘best’, FCI, and ‘worst’, HF, are irrefutable, but the intermediate methods are less clear and depend on the type of chemical problem being addressed.14 The use of HF in the case of FCI was due to the computational cost. The molecular Hartree-Fock wave function is written as an antisymmetrized product (Slater determinant) of spin-orbitals, each spin-orbital being a product of a spatial orbital
and a spin function (either α or β). The expression for the Hartree-Fock molecular electronic energy
is given by the variation theorem as
where D is the Slater-determinant Hartree-Fock wave function and
and
are given by
Since
does not involve electronic coordinates and D is normalized, we have
. The operator
is the sum of one-electron operators
and two-electron operators
we have
where
and
The Hamiltonian
is the same as the Hamiltonian
for an atom except that
replaces
in
Hence
where
And
can be used to give
Therefore, the Hartree-Fock energy of a diatomic or polyatomic molecule with only closed shells is
and
where the one-electron-operator symbol was changed from
to
5
Mulliken load
Mulliken's loads are derived from the Mulliken population analysis and provide means for estimating partial atomic charges from numerical chemistry calculations, particularly those based on the linear combination of atomic orbitals. If the coefficients of the basic functions in the molecular orbital are Cμi for μe the basic function ie in the orbital molecular, the coefficients of the density matrix are:
for a compact closed system in which each molecular orbital is doubly occupied. The population matrix P therefore has the following coefficients:
S is the overlay matrix for basic functions. The sum of the set of terms of
is N - the total number of electrons. The Mulliken population analysis aims first of all to distribute the N electrons on all the basic functions. This is done by taking the diagonal elements of
and factorizing the non-diagonal elements equally between the two appropriate basic functions. Non-diagonal terms including
and
this simplifies the operation to a sum on a line. This defines the gross orbital population (GOB) as:
The terms
lie on N and then divide the total number of electrons between the basic functions. It then remains to sum these terms on all the basic functions of a given atom A in order to obtain the gross atomic population (GAP). The integral of the GAPA terms also gives N. The load, QA, is then defined as the difference between the number of electrons on the free isolated atom, which is the atomic number ZA, and the raw atomic population:
The problem with this approach is the even distribution of non-diagonal terms between the two basic functions. This leads to charge separations between the molecules that are exaggerated. Many other methods are used to determine atomic charges in molecules.21,22 Concerning the nuclear contribution, the nuclear charge
can be written as
where
and
account for the Mulliken net and gross atomic charge.21 According to the Mulliken population analysis, the nuclear charge for A can be written as
which upon substitution in the dipole moment expression yields
Note that
and
so that
where
and
The first two terms in eq. for
are of atomic origin where the first one, involving the net atomic charge, is the only term with a classical counterpart. The second term resembles Coulson’s atomic dipole, and the integral
is the distance from the centroid of the hybrid orbital to nucleus A. For the third term, the integral
is the distance of the center of charge from the midpoint of the chemical bond A-B. This contribution to the dipole moment has been referred to as the homopolar dipole21 by Mulliken. As can be seen, the dipole moment has been partitioned into three contributions: the net atomic charge, the atomic dipole, and the homopolar dipole. Since the density matrix is invariant with respect to the choice of origin and since the sum of all net atomic charges vanishes, this partitioning of the dipole moment does not depend on the choice of origin for the system.5,23
Atomic polar tensor (APT)
One of the most useful methods for interpreting and predicting infrared intensities comes from the atomic polar tensor (APT) formalism.24,25 In the APT framework, the derivative of the molecular dipole moment vector with respect to the ith normal coordinate (which is directly related to the infrared intensity of the ith fundamental mode), can be expressed as
For each atom α in molecule, the quantities
where
and
form the APT, represent by a
matrix
So, if all the experimental infrared intensities and normal coordinates are known as well as the permanent dipole moment for a given molecule, the APT can be determined. On the other hand, these APTs can also be calculated by the SCF method and used to predict infrared intensities. These intensities can then be interpreted by partitioning the APT. This has been done before in the "charge-charge flux-overlap" (CCFO) model, first introduced by King and Mast26,27 and later applied by Person et al.28
The general expression for the APT is:
where E is the identity matrix and each term of the APT is represented by a 3 X 3 matrix. The four contributions in the above equation can be identified according to Person, Coulson, and Mulliken terminology as charge, charge flux, atomic dipole flux, and homopolar dipole flux. Comparing with the CCFO model, the difference introduced in this work lies in the fact that the overlap term has been decomposed into two flux contributions (atomic dipole and homopolar dipole fluxes).
In eq. for
the first two terms are the only classical contributions, one of them being the Mulliken net charge of atom a in its equilibrium position,
and the other being the "charge flux" corresponding to charge migration as the chemical bond involving the α atom has been distorted. The sum over all atoms, A, implies there is electronic density deformation involving all the atoms in the molecule. These two terms have already been well discussed by Person, Zilles, and other28,29,30 The atomic dipole flux can be separated into two parts if the gradient of the density matrix and center of charge integrals are taken inside the parentheses:
and
the first of the two terms in equation
involves only the atom for which the APT is being calculated because only these
depend on
Hardware and software
For calculations a computer models was used: IntelÒ CoreTM i3-3220 CPU @ 3.3 GHz x 4 processors,31 Memory DDR3 4 GB, HD SATA WDC WD7500 AZEK-00RKKA0 750.1 GB and DVD-RAM SATA GH24NS9 ATAPI, Graphics Intel Ivy Bridge.32 For calculations of computational dynamics, the Ubuntu Linux version 16.10 system was used33 and the software used for the molecular dynamics was GAMESS.16,34
The Figure 2 show on cell structure of a protonated rhodochrosite crystal of structure Stoichiometric is CH19Mn6O8, obtained after molecular dynamics via unrestricted Hartree-Fock method, in basis set CEP-4G, CEP-31G and CEP-121G.35–96 The Figure 3A–D) show the normalized absorption spectrum as a function of the vibrational frequencies of the protonated rhodochrosite crystal for UHF-CEP-4G basis set, UHF-CEP-31G and UHF-CEP-121G. The rhodochrosite crystal unit cell of structure CMn6O8, where the load distribution by the molecule was verified in the unrestricted Hartree-Fock method, UHF CEP-4G (Effective core potential (ECP) minimal basis), UHF CEP-31G (ECP split valance) and UHF CEP-121G (ECP triple-split basis), through the analysis of APT and Mulliken loads.97–102 The rhodochrosite unit cell was protonated, then presented the structure CH19Mn6O8 for the study with ab initio methods with +4 multiplicity. The displacement of charges by the molecule was analyzed to verify the site of molecular action. The load distribution by the protonated crystal is evaluated in Table 1, and its vibrational frequencies in Table 2. The Table 2 show the maximum absorbance peaks in the CEP-4G, CEP-31G and CEP-121G set basis are present at the frequencies 2172.23 cm-1, with a normalized intensity of 65%; 2231.4 cm-1 and 45.4%; and 2177.24 cm-1 and 100%, respectively.
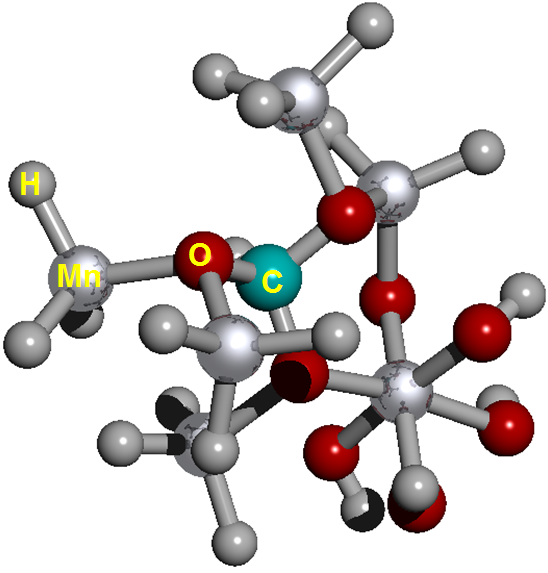
Figure 2 Cell structure of a protonated rhodochrosite crystal. Represented in red the oxygen; silver in color Manganese; in gray color Hydrogen; in light see green color the Carbon. Stoichiometry: CMn6O8. Stoichiometry protonated: CH19Mn6O8.
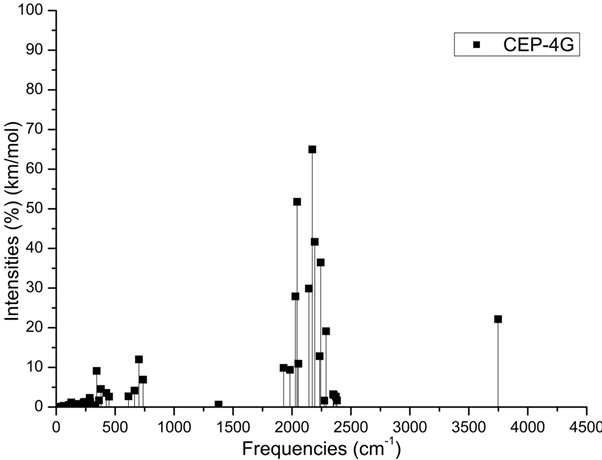
Figure 3A Absorbance spectrum plot as a function of vibrational frequencies of protonated rhodochrosite crystal for UHF-CEP-4G basis set.

Figure 3B Absorbance spectrum plot as a function of vibrational frequencies of protonated rhodochrosite crystal for UHF-CEP-31G basis set.
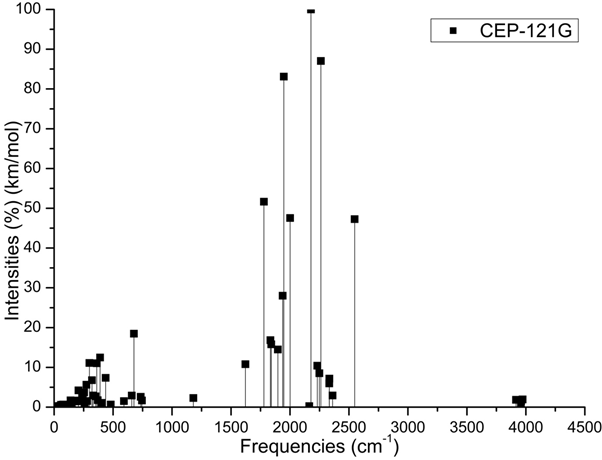
Figure 3C Absorbance spectrum plot as a function of vibrational frequencies of protonated rhodochrosite crystal for UHF-CEP-121G basis set.
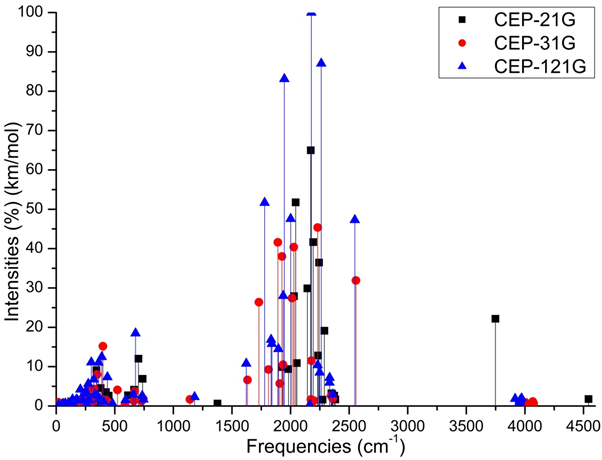
Figure 3D Absorbance spectrum plot as a function of vibrational frequencies of protonated rhodochrosite crystal for UHF-CEP-4G basis set, UHF-CEP-31G and UHF-CEP-121G.
|
Basis Sets
|
Mulliken
|
|
|
APT
|
|
|
| |
Charge*
|
|
δ
|
Charge*
|
|
δ
|
|
CEP-4G
|
-1.064
|
1.064
|
2.128
|
-1.366
|
1.366
|
2.732
|
|
CEP-31G
|
-1.034
|
1.034
|
2.068
|
-1.362
|
1.362
|
2.724
|
|
CEP-121G
|
-1.325
|
1.325
|
2.65
|
-1.461
|
1.461
|
2.922
|
Table 1 Load shifting on given basis sets of the Mulliken and APT method
*±1,602 176 634×10−19 C (Coulomb).
| |
ν (cm-1)
|
I (%)
|
ν (cm-1)
|
I (%)
|
ν (cm-1)
|
I (%)
|
ν (cm-1)
|
I (%)
|
|
CEP-4G
|
2172.23
|
64.9904
|
2043.25
|
51.7671
|
2193.1
|
41.6608
|
2242.97
|
36.4643
|
|
CEP-31G
|
2231.4
|
45.3589
|
1891.26
|
41.6207
|
2027.77
|
40.3978
|
1926.32
|
38.0064
|
|
CEP-121G
|
2177.24
|
100
|
2261.98
|
87.0553
|
1947.03
|
83.1151
|
1778.57
|
51.6624
|
Table 2 Peaks maximum absorption intensity by the frequency given. Absorbance frequency as a function of vibrational frequencies of protonated rhodochrosite crystal for UHF-CEP-4G basis set, UHF-CEP-31G and UHF-CEP-121G
ν=Frequency (cm-1); I=Normalized Intensity (%).
Analysis
The Mulliken load method in the UHF-CEP-4G base set; UHF-CEP-31G and UHF-CEP-121G are sufficient to show that the sites of action of the rhodochrosite crystal structure are found in three Oxygen-linked Manganese atoms, which are attached to the central Carbon atom, as well as these. Oxygen atoms and the central Carbon. These Manganese atoms show a slight negative to neutral load shift in the CEP-4G set basis, neutral to positive in the CEP-31G and CEP-121G set basis at the Mulliken charges, (Figure 4). The charge displacement is strong in the oxygen atoms, especially those near the central carbon, with negative load in all set basis studied, both in the APT and Mulliken charges. The central carbon atom on all set basis is positively charged in both APT and Mulliken load, except Milliken in CEP-31G, which is neutral. As might be expected from the charges by APT, the strong positive load manganese atoms, the strong negative load oxygen, the positively charged carbon atom. The manganese atom farthest from the carbon atom has a slight positive to neutral load shift. The Mulliken load method presents a better result when compared to the APT, in the studied set basis, for protonated rhodochrosite crystal, with a smaller load variation δ=2,650 u.a for CEP-121G. The absorption peaks are in a Gaussian between the frequencies 1620 cm-1 and 2520 cm-1, Figure 3D. The largest load variation in the APT and Mulliken methods were obtained in the CEP-121G base set, with δ=2.922 e δ=2.650, respectively, being δAPT > δMulliken, in all sets of calculated basis, (Table 1).

Figure 4 UHF-CEP-4G; UHF-CEP-31G and UHF-CEP-121G for APT and Mulliken.








