eISSN: 2576-4543


Research Article Volume 8 Issue 4
1Master en Ciencias, Ingeniero Mecánico, Profesor Instructor, Centro Nacional de Electromagnetismo Aplicado, Universidad de Oriente, Cuba
2Departamento de Telecomunicaciones, Facultad de Ingeniería Eléctrica, Universidad de Oriente, Cuba
3Master en Ciencias, Ingeniero en Telecomunicaciones, Profesor Auxiliar, Departamento de Telecomunicaciones, Facultad de Ingeniería Eléctrica, Universidad de Oriente, Cuba
4Master en Ciencias, Licenciado en Mecánica, Profesor Auxiliar, Centro Nacional de Electromagnetismo Aplicado, Universidad de Oriente, Cuba
5Doctor en Ciencias Biológicas, Licenciado en Física, Profesor Titular, Centro Nacional de Electromagnetismo Aplicado, Universidad de Oriente, Cuba
Correspondence: Leonardo Mesa Torres Master en Ciencias, Ingeniero Mecánico, Profesor Instructor, Centro Nacional de Electromagnetismo Aplicado, Universidad de Oriente, Santiago de Cuba 90400, Cuba
Received: December 16, 2024 | Published: December 24, 2024
Citation: Torres LM, Delgado IG, Calzado EM, et al. Influence of inter-and inter-pair distance of electrode arrays on tumor treatment. Phys Astron Int J. 2024;8(4):246-249. DOI: 10.15406/paij.2024.08.00356
Electrochemical therapy (EChT) is used for the treatment of cancer. The application of this therapy is simple, safe, effective and induces minimal adverse events in the organism. The objective is to evaluate the induced effectiveness of the distance of the electrode arrays (single and multiple pairs) for the quantification of the percentage of tumor destruction (PDT). A computer program was used for the calculation and simulation of PDT and a sample of (24 male and 24 female) BALB/c/Cenp laboratory mice. It is concluded that this therapy is effective for the treatment of tumors since the percentage of tissue damage increases; by more than 80% at a distance (0.5 ≤ d ≤ 1) cm from the electrode arrays inserted perpendicularly in the tumor geometry, which allows its use for the treatment of different types of tumors.
Keywords: tumor treatment, electric field, electrode arrays, electric field, tissue damage
Electrolytic ablation therapy or electrochemical therapy (EChT) consists of the application of direct electric current of very low intensity to the tumor by means of the insertion of two or more electrodes in its interior and/or vicinity.1 The first experiences in Cuba on the use of EA on tumors were reported in.2,3 In recent years, the use of different types of physical anti-cancer therapies has become increasingly popular, such as electroporation,1s hyperthermia,2 laser,3 electrochemical therapy (EChT)4,5 and E2 therapy (EChT+electroporation).6 EChT is simple, safe, effective, and induces minimal adverse events in the organism and constitutes another option when established anti-cancer therapies (surgery, radiotherapy, chemotherapy and immunotherapy) fail or cannot be applied due to the patient's general condition; therefore, it can be used prior to surgery to reduce the size of the tumor and combined with radiotherapy and chemotherapy, which reduces the doses usually used by these conventional therapies by 10%.7 However, two studies.8,9 report the death of several BALB/c/Cenp mice carrying highly aggressive and metastatic F3II mammary carcinoma. The death of these animals is essentially due to metastasis in the lung and other organs (liver and kidney) and to a lesser extent to EChT; since their overall effectiveness (partial remission + complete remission) is greater than 60%, in agreement with preclinical,10,11 and clinical results.7 This type of antitumor treatment has not been as well recognized and standardized as the established oncospecific therapies mentioned above and its mechanism of action is poorly understood, despite these deaths. The non-standardization of this type of therapy is due to the fact that the optimal dose and configuration of electrodes (single and multiple pairs) inserted into the tumor geometry have not been established.5-9 Long experimentation times and considerable material resources are required to understand these aspects if they are undertaken only from the experimental point of view. Therefore, physical-mathematical modeling is suggested as a fast and feasible way.8,9 The Cuban Bioelectricity group focuses its efforts in proposing different two-dimensional (2D),12-15 and three-dimensional (3D),16,17 physical-mathematical models that allow knowing the spatial distributions of the electric potential (Ф), the intensity of the electric field (E), electric current density (J), temperature (T), pH fronts, and the percentage of PDT tissue damage generated by different geometries of single electrodes,12,13,15-17 or multiple pairs of electrodes inserted collinearly or not in the tumor.14 Experimental results in in vitro, preclinical and clinical studies, and simulations obtained in current mode of EChT are similar to those in electrical voltage mode. The electric current mode consists in that the intensity of the direct electric current remains constant during its application; however, the electric voltage changes due to the variation of the electrical resistance of the tumor. The electrical voltage mode consists in that the electrical voltage remains constant during its application; however, the intensity of the electrical current changes due to the variation of the electrical resistance of the tumor; in both modes of EChT can be explained by the toxic products produced by electrochemical reactions, tissue damage (by apoptosis and necrosis) and other biophysical-chemical processes that are induced in the tumor.17,19-23 It is important to note that the bioeffects and efficacies/effectivities are similar when both modes of EChT are used in mice10 and humans.17 Amount of electric charge (Ф) 80 C/cm3 and potential difference (ΔVo) between 10 and 12 V are the doses that induce the highest antitumor efficacies on different tumor types when EChT is used in current and voltage-gated mode, respectively.14 These electrode configurations have been experimentally validated in tumors,7-10, 18,19 potato,13,20 in silico,13 and in vitro.21 Calzado et al.14 as they report the spatial distributions of electric potential (Ф), electric field strength (E), electric current density (J), temperature (T), pH fronts, on tumor geometry; Currently, in the scientific literature there are no reports of the influence of the distance (d) of single and multiple pair electrode array configurations on the (PDT).
In this study, the following were used forty-eight BALB/c/Cenp male and female mice (24 males and 24 females), 6–7 week old and 18–20g mass, supplied was provided by the (CENPALAB, Havana, Cuba), were used, (Registration number 16/17, code AETM0917, 17 May 2017), guidelines animal ethic comission of República de Cuba and Council Directive 86/609/ECC of 24 November 1986, which followed guidelines for the welfare of animals in experimental neoplasia con platinum needle electrodes are inserted parallel to the z axis. ONCOCED B&E-01 electrostimulator designed and built by researchers at Centro de Biofísica Médica, Centro Provincial de Electromedicina and Centro Nacional de Electromagnetismo Aplicado (CNEA), all from Santiago de Cuba, Cuba. was used for DC treatment. This equipment was calibrated with a calibrator (Bodystat15-MDD device, Tampa, FL) before, during, and after DC treatment to evaluate its stability. This calibrator (courtesy of Ing. Antonio Gómez Yépez, Grupo de Terapia Metabólica, Veracruz, México) had an electric resistance of 500±0.1V [Lafargue et al., 2013]. The electrical voltage (in V), DC intensity (in mA), and electrical charge (in C) were monitored every 2min during treatment. Stainless steel electrodes of 0,5 ≤ d ≤ 1mm diameter and 68.5mm long were used. Electrode material was AISI 316L austenitic stainless steel (Jiangsu Shenghengte Stainless Steel, Wuxi, China).
In the Figure 1 shows the single and multiple pair electrode arrays used in the experiment.
Figure 2 shows the studies performed for the configuration of collinear electrodes inserted perpendicularly to the tumor at a distance d = 0.5; 0.7 and 1.0 cm called: b) Ci-Ia, Ci-Ib, c) Ci-Ic and d) Cp-II and multiple pairs of electrodes inserted concentrically at 45; 135; 225 and 3150 respectively, with respect to the x-axis.13,14
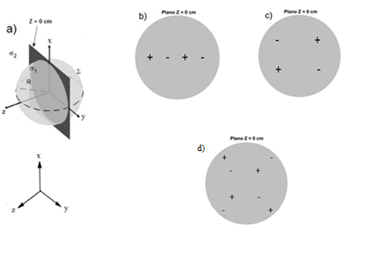
Figure 2 Different electrode configurations inserted in the tumor. a) Schematic representation of a spherical tumor. Electrode configurations: b) Ci-Ia, Ci-Ib, c) Ci-Ic, d) Cp-II
A group of multiple anodes and cathodes inserted individually and perpendicularly along the tumor diameter of radius R = 2.5 cm separated at a distance (d) of 0.5, 0.7 and 1 cm, named Ci-Ia, Ci-Ib and Ci-Ic, respectively; and the concentric one, with the electrodes located at 45, 135, 225 and 315°, referenced as Ci-II, were simulated. The configurations of multiple straight needle electrodes were shown in Table 1 (Configurations Ci-Ia, Ci-Ib, Ci-Ic and Ci-II). The insertion depth of the electrodes was along the z = 0 axis.
|
Electrode arrangement |
Parameters |
||
|
Quantity |
Polarity |
Positioning |
|
|
Ci-Ia |
4 |
Electrodes 1 and 3 (positive) and electrodes 2 and 4 (negative) a |
Collinear electrodes, d = 0.5 cm. |
|
Ci-Ib |
4 |
Electrodes 1 and 3 (positive) and electrodes 2 and 4 (negative) a |
Collinear electrodes, d = 0.7 cm. |
|
Ci-Ic |
4 |
Electrodes 1 and 3 (positive) and electrodes 2 and 4 (negative) a |
Collinear electrodes, d = 1 cm. |
|
Ci-II |
4 |
Electrodes 1 and 3 (positive) and electrodes 2 and 4 (negative) b |
Concentric electrodes 1; 2; 3 and 4 placed at 45; 135; 225 and 315°, respectively, with respect to the x-axis. |
|
Cp-I |
|
Electrodes 1 and 3 (positive) and electrodes 2 and 4 (negative) b |
Pairs of colonial electrodes, d = 1 cm |
|
Cp-II |
8 |
Electrodes 1; 3; 5 and 7 (positive) and electrodes 2; 4; 6 and 8 (negative) b |
Concentric electrode pairs 1-2; 3-4; 5-6 and 7-8 placed at 45; 135; 225 and 315° with respect to the x-axis, respectively. |
Table 1 Parameters for configurations Ci-Ia, Ci-Ib, Ci-Ic, Cp-I and Cp-II
The calculation and simulation of the percentage of tissue damage (TDPi, in %) that is induced in the tumor (i = 1) and in the surrounding healthy tissue (i = 2) was performed by the following equation.
(1)
where NDi is the number of points satisfying the condition T ≥ 42 oC and NTi the total number of points in the tumor (i = 1) and in the surrounding healthy tissue (i = 2)). NT is calculated in the entire tumor volume of R = 2.5 cm (NT1 = 42 025 points) and R = 5 cm (NT1= 84 050 points). In addition, NT is calculated in the surrounding healthy tissue comprised in a spherical cap between R = 2.5 (NT2 = 17 651 points).
In the figure 3. shows the spatial pattern of tissue damage generated by collinear electrode configurations Ci-Ia (a), Ci-Ib (b), Ci-Ic (c) and multiple pairs of electrodes Cp-I (d); at distance d = (0.5, 0.7,1.0) cm.
Figure 4 shows the spatial pattern of tissue damage generated by single electrode (Ci-II) and multiple concentric electrode pairs (Cp-II) configurations placed at 45; 135; 225 and 315o inserted perpendicularly in the tumor geometry (d), with respect to the x-axis, respectively. The parameters of this configuration are presented in Table 1.
Figure 3 showed that the highest PDT distribution was generated by the Ci-Ib configuration at a distance d = 0.7 cm and Cp-I, at d = 1 cm; for a tumor radius R = 2.5 cm. in agreement with the results of Calzado et al.13 Multiple pairs of electrodes behave like multiple single electrodes when d > 1 cm, which induce the greatest growth regression and complete remission of F3II mammary carcinoma and survival of male and female BALB/c/Cenp mice.8 The other reason is that the anodes (positive electrodes) inserted into the tumor attract the cancer cells, which are negatively charged.14,25 Moreover, it is perceived that when R increases the region of induced destruction is smaller. In figure 4 it was demonstrated that the Ci-II and Cp-II configurations at 45; 135; 225 and 315o, have around 80% percent of tumor destruction and with the minimum damage to the surrounding healthy tissue; because the temperature (41-45)oC generated by these configurations is induced in the whole volume of the tumor; according to the theory,9,14 (results not shown in this study); which corroborates, that it is not only the intensity of the electric current. The (EChT in current mode), the intensity of the electric field (EChT in voltage mode) and the temperature are the essential responsible for tissue damage in tumors, but the toxic products, coming from the electrochemical reactions. Therefore, the most accepted antitumor mechanism of EChT is the electrochemical one, in agreement with what has been documented in the literatura.7-11,13,18-23,36,37 Therefore, alterations induced by this therapy in healthy mice are reversible. It promotes a significant delay in the growth of highly invasive and metastatic tumors, since the spatial pattern of tissue damage adopts the same shape as the electrode array and the insertion of multiple concentric straight needle electrodes along the depth and radial direction of tumor growth can enhance the effectiveness of this therapy, as demonstrated experimentally in.38
The lowest PDT values are obtained in the configurations of electrodes inserted individually in the tumor, with a lower percentage Ci-Ia; with respect to Cp-II; for a separation between the electrodes of 0.7 cm. The Ci-II and Cp-II electrode configurations are the ones that induce the highest tissue damage values and therefore can be used for the treatment of large and deep tumors, which is of vital importance to improve the geometric description of the electrode array and increase the effectiveness in the treatment of tumors.
The authors thank the technicians and other colleagues from the National Center for Applied Electromagnetism, the Center for Medical Biophysics and the Faculty of Mechanical Engineering who made this work posible.
The authors declare that there are no conflicts of interest.

©2024 Torres, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.