eISSN: 2377-4304


Case Report Volume 15 Issue 5
Gyneacologic Department, University Hospital of Bellvitge, IDIBELL (Bellvitge Biomedical Research Institute), Spain
Correspondence: Castilla Rodríguez M. Gyneacologic Department University Hospital of Bellvitge (Hospitalet de Llobregat, Barcelona), Spain
Received: September 18, 2024 | Published: October 24, 2024
Citation: Castilla RM, García TA, Martínez GJM, et al. Uterine fibroid metastasis as the initial manifestation of lobular breast cancer. Obstet Gynecol Int J. 2024;15(5):238-241. DOI: 10.15406/ogij.2024.15.00765
The occurrence of breast cancer metastasis in a uterine leiomyoma is exceptional, and its diagnosis prior to detection of the primary tumor is even rarer. We describe at case of a 52-year-old female patient with a large fast growing uterine fibroid that caused pelvic pain and abdominal distention. A total abdominal hysterectomy with bilateral salpingo-oophorectomy was performed. Histopathology of the surgical specimen showed a characteristic pattern of invasive breast lobular carcinoma in the leiomyoma.
A magnetic resonance image of the breast was then performed, which reveled primary and bilateral breast cancer. She subsequently underwent neoadjuvant endocrine therapy plus cyclin inhibitors, breast-conserving surgery, and radiotherapy. To date, the patient is alive and asymptomatic after 3 years of follow-up.
Keywords: uterine fibroid, breast cancer, metastasis, pelvic pain
Metastasis of an extragenital tumor in the female genital tract is an exceptional entity, and it is even rarer to be diagnosed prior to detection of the primary tumor. Those tumors that have been characteristically related to this dissemination have their origin at the mammary, gastrointestinal, lung, kidney and skin levels in order of frequency,1 with the breast being the most common primary tumor.2
The organs most affected by metastasis within the genital tract are the ovaries and vagina. Invasion is found much less frequently in the uterus, although up to 8% of cases of breast cancer metastases will implant in this location, preferably in the myometrium.3 It should be noted that lobular breast cancer is the most common type of carcinoma that metastasizes to the uterus.3
We present a clinical case of incidental detection of a breast cancer metastasis in a uterine leiomyoma with the aim of reviewing atypical locations of the breast metastases, analyzing the suspected diagnosis, the pathologic anatomy of the uterine leiomyoma, and highlighting the prognostic impact of the finding, which undoubtedly influences the therapeutic approach.
We present a 52-year-old premenopausal woman (gravida 3, para 2, mis-birth 1) with arterial hypertension under medical treatment. She was referred to our center in February 2021 in the context of uterine leiomyomatosis referring chronic pelvic pain, weight sensation in hypogastrium and urgency urinary incontinence of one year of evolution.
Transvaginal ultrasound showed a 11x10x9 cm uterine tumour suggestive of subserous myoma FIGO 7, located in the left lateral fundus of the uterus (Figure 1). Reviewing her medical history, we found a computed tomography scan 5 years earlier with a 4 cm uterine fibroid in the same location (Figure 2).
Due to the increase in the size of the uterine fibroid, as well as the patient's symptoms, a decision was made to perform surgical treatment consisting of a simple total abdominal hysterectomy with bilateral salpingectomy, which was carried out on 3 June 2021 without any complications. The specimen weighed 1315.60 g.
In the anatomopathological study, the macroscopic aspect of the uterus was normal (Figure 3), with several subserous nodular structures, the largest of 11 cm in diameter, whitish and fasciculated in appearance, of elastic consistency with focal yellowish areas compatible with uterine myoma.
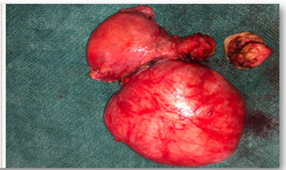
Figure 3 Myomatous uterus, subserosal myoma of 11 cm in greatest
diameter.Myomatous uterus, subserosal myoma of 11 cm in greatest diameter.
Microscopic examination of the uterine myoma revealed atypical cells showing a linear arrangement characteristic of invasive breast lobular carcinoma. Immunohistochemical staining of the tumor was positive for estrogen receptor, progesterone receptor and cytokeratin AE1/AE3, also suggestive of mammary origin.
The anatomic pathology report confirmed the histological types of breast cancer
Both tumors with disease free margins.
After the diagnosis of uterine metastasis of probable mammary origin, mammography and breast ultrasound were performed, detecting two suspicious tumors (BIRADS 4C) of 12 mm, one in each breast (Figure 4).
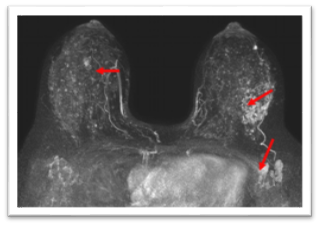
Figure 4 Right breast: Focal non mass enhancement in UCS of 15 mm
Left breast: Extensive non-mass enhancement in SCU of 49 mm
Left axilla: 3 suspicious left axillary nodes
The core needle biopsy (CNB) of the right breast was compatible with infiltrating ductal carcinoma and that of the left breast with invasive lobular carcinoma. Both tumors were estrogen and progesterone receptor positive, HER2NEU negative and Ki67 20% and 13% respectively.
The right axillary ultrasound showed no suspicious lymphadenopathies but at least 3 lymphadenopathies were visualized in the left axillary ultrasound. Fine needle puncture (FNA) of these lymphadenopathies was performed and the cytological study was compatible with reactive lymphadenitis without evidence of neoplasia, so a BAG of these lymphadenopathies was performed with positive result for lobular carcinoma.
The local assessment of primary disease at the breast level was complemented with a magnetic resonance imaging (MRI) that showed a 15 mm focal non-mass enhancement in the right breast and extensive segmental non-mass enhancement of 49 mm in the left breast consistent with the lesion already biopsied (BIRADS 6), as well as suspicious-looking left axillary nodes (Figure 5).
The study of the extension of the disease included a Positron Emission Tomography (PET CT) where the only relevant finding was discreetly irregular activity in the breast with no evidence of other deposits suggestive of distant metastasis.
With the diagnosis of synchronous bilateral breast neoplasia (clinical staging cT1N0 in right breast and cT1N1 in left breast) M1 (single uterine metastasis) is considered subsidiary of neoadjuvant treatment with cyclin inhibitors (ribociclib) and double hormonal blockade (letrozole and gonadotropin-releasing hormone analogues) which began on 15/07/2021.
After six months of systemic treatment, a new MRI of the breast was performed, showing a complete radiological response (Figure 5).
On 04/25/22, breast surgery was performed consisting of bilateral breast conserving surgery associated with oncoplasty techniques (bilateral reduction pattern), selective biopsy of the right sentinel lymph node and left axillary lymphadenectomy.
The two sentinel lymph nodes removed in the right axilla were negative for tumor cells. In the left axillary lymphadenectomy, 23 nodes were analyzed, of which 9 were positive, 4 macrometastases, 3 micrometastases and 2 isolated tumor cells. Consequently, the definitive staging of the disease on the right side was ypT1N0 and on the left side ypT2N2aM1.
As local adjuvant treatment, external radiotherapy has been performed on the left breast and ipsilateral axillary lymph node areas III, supraclavicular and internal mammary chain (CMI) and on the right breast at a dose of 40.05 Gy to 2.67 Gy/s.
At the systemic level, the treatment already started pre-surgery with cyclin inhibitors and double hormonal blockade is maintained. Currently, the patient remains free of disease 1.5 years after diagnosis.
The finding of metastatic breast cancer within a uterine myoma is exceptional.4,5 Even so, after this review, we consider it essential to conduct a thorough differential diagnosis when faced with a uterine mass presenting with acute symptoms (such as pelvic pressure or abdominal pain, abnormal uterine bleeding, and compression symptoms) and rapid growth. Although ultrasound has not proven to define the uterine tumour with sufficiently high sensitivity and specificity, the ultrasound characteristics of uterine myomas can help us determine the degree of suspicion regarding the malignancy of a tumour. A uterine tumour with predominantly central and abundant Doppler uptake that doubles in size between two gynaecological check-ups should prompt us to rule out, albeit infrequently, a malignant nature of the tumour, such as a uterine sarcoma. Other entities to include in the differential diagnosis would be focal adenomyosis, endometrial polyps, or benign ovarian tumours such as fibroma or thecoma. As a secondary line of differential diagnosis, given its much lower frequency but significant impact on the patient's prognosis, we should also consider the possibility of metastasis as the origin of a uterine myoma that is growing and beginning to present symptoms, as is the case with our patient.
One of the longest series on metastatic tumors in the female genital tract described in the literature by Mazur et al.6 includes 149 cases where the ovary and vagina were the most frequent sites of metastasis (75.8% and 13.4% respectively), and only 8.1% were to the uterus (4.7% uterus, 3.4% endometrium, 3.4% cervix). Breast cancer appears as the second most common primary tumor site after gastrointestinal; of the 52 cases of metastatic breast cancer in gynecological organs recorded, 88.5% involved the ovaries, 5.8% the vagina, 3.8% the endometrium, and 1.9% the vulva, with no reference in any case to metastasis within a uterine myoma, hence the relevance in incorporating our case to the current literature.
The signs and symptoms described in the literature related to the presence of metastasis in a uterine myoma are meno-metrorrhagia (6/13, 46.2%), rapid growth of the uterus (4/13, 32.5%) and asymptomatic (3/13, 23.1%).3 Similarly, our patient presents weight sensation in the hypogastrium and urinary incontinence, symptomatology associated with myoma enlargement due to compression of neighboring structures.
We have only found two cases similar to ours described in the literature, in which the diagnosis of uterine metastasis of a breast carcinoma was the first diagnosis, after its detection in the anatomo-pathological study of the uterine myoma, with an undetected primary breast.5,7
Minelli et al.7 describe a 37-year-old patient with a uterine myoma and hypermenorrhea. Given the age of the patient, the symptomatology associated with myoma growth could be attributed to a hormonal component, being less likely to think of an underlying neoplasm that justifies such growth, as was the case.
In our case, taking into account the normal breast screening controls to date, with a family history with no history or other relevant associated symptomatology, no complementary study prior to surgery seemed to be indicated. However, it was a known uterine myoma, which in recent weeks began with symptoms attributed to extrinsic compression of the myoma to adjacent neighboring organs and its growth was observed in the ultrasound control performed.
This symptomatology should lead us to suspect as an underlying cause a degeneration of the myoma or, even assuming the low probability of this, a neoplastic origin that justifies the abnormal growth of the uterine mass.
Finally, in reference to the most frequent histological types of breast cancer, we find, in first place, non-special invasive carcinoma, ductal type (70%) and, in second place, invasive lobular carcinoma (5-15%).8
However, it is the infiltrating lobular carcinoma that is most likely to metastasize to the female genital tract.9,10 Reviewing the histological types of breast cancer in the Razia et al. report, ductal carcinoma comprised 61.55% (8/13) and lobular carcinoma 38.4% (5/13) of metastases to uterine leiomyoma.3 In our case, the patient was diagnosed with both ductal and lobular breast carcinoma, but only lobular carcinoma metastasized to the uterine leiomyoma.
Metastasis of a breast carcinoma to a uterine myoma is a very rare entity and is diagnosed by surgical specimen finding. The symptomatology of this entity is nonspecific, although the rapid growth of a uterine myoma should alert us to rule out any malignant pathology associated with it, both in the form of metastasis and uterine sarcoma. We consider it especially important to consider this option given the prognostic impact it will have on these patients.
None.
None.
Authors declare that there is no conflict of interest.

©2024 Castilla, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.