eISSN: 2377-4304


Research Article Volume 15 Issue 3
1Department of Endocrinology, University College Hospital London, NW1 2BU, UK
2Department of Medical School, University College London, London, WC1E 6BT, UK
3Department of Andrology, University College London, London, WC1E 6BT, UK
4Institute for Women’s Heath, University College London, UK
Correspondence: Anastasia Dimakopoulou, Department of Endocrinology and Diabetes, University College London Hospital, 235 Euston Road, London, NW1 2PG, UK
Received: June 01, 2024 | Published: June 12, 2024
Citation: Dimakopoulou A, Walker A, Pahariya P, et al. Spermatogenesis induction audit over a 5-year period at a UK male fertility centre. Obstet Gynecol Int J. 2024;15(3):137-141. DOI: 10.15406/ogij.2024.15.00749
Background: This audit aimed to evaluate and measure the outcomes of spermatogenesis induction over a 5-year period. Men with primary, as well as central hypogonadism, received gonadotrophin therapy to stimulate sperm production and fertility outcomes, including live birth rates. Predictors associated with live births, were measured retrospectively.
Methods: Men with severe oligospermia (sperm concentration <5million/ml), having gonadotrophin prescriptions for a minimum of 6 months, were identified via the electronic prescription system. They were asked to complete a service evaluation questionnaire and their electronics records were reviewed.
Results: Men with persistent azoospermia were more likely to have a diagnosis of PH (Odds ratio 22.5, p<0.001) and smaller testicular size (Odds ratio 8.8, p<0.001), compared to men with successful spermatogenesis. Twenty-eight per cent (13/47) had partners, who conceived spontaneously and delivered healthy babies. Nine per cent (4/47) had a live birth after ART. Live birth rate was higher in men with CH compared to PH, with 17 of 45 (38%) men with CH having a partner that successfully delivered a baby.
Conclusion: Men with mainly central hypogonadism and female partners with no known subfertility are most likely to achieve conception and live birth. Patient education on the results of semen analysis or female factors affecting fertility could improve overall outcomes.
Keywords: spermatogenesis, semen analysis, primary hypogonadism, central hypogonadism, assisted reproduction therapy
SA, semen analysis; PH, primary hypogonadism; CH, central hypogonadism; ART, assisted reproduction therapy
One in seven couples in the UK are affected by infertility, which is attributed to male factors in 50% of cases.1 Up to half of the cases of male infertility are deemed idiopathic with no effective treatment available for them.2 However, the remaining cases are attributed to primary hypogonadism (PH), such as Klinefelter syndrome (KS), or central hypogonadism (CH), such as congenital hypogonadotrophic hypogonadism (CHH) or pituitary disease.
Men with PH or CH, seeking fertility are prescribed gonadotrophins and they encouraged to conceive naturally, as sperm production is induced3 and sperm concentration increases in their semen analysis (SA). In our centre, if there is no positive pregnancy test after a minimum of 12 months on active gonadotrophin titration, the couple are asked to consider intrauterine insemination (IUI), in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) therapy. The decision depends on SA result, the female partner’s age and ovarian reserve.
Although treatment pathways for hypogonadal men with infertility have been established over the last thirty years,4 the effectiveness of such pathways is diverse and data from large studies are scanty. Data focused on spermatogenesis induction and subsequent fertility outcomes are less frequently published, compared to female fertility data. Also, the male patients’ perspective on the provided fertility services has not been recorded. The aim of this study was to audit practice in a dedicated Male Fertility clinic, in terms of spermatogenesis rates and fertility outcomes, along with a patient centred questionnaire evaluating the provided service.
Study design and participants
Gonadotrophin prescriptions, distributed from the Fertility clinic, from Jan 2017 to December 2021, were identified via the electronic prescription system. A total of 168 patients had spermatogenesis induction with gonadotrophins for a minimum of 6 months, in the context of primary or central hypogonadism. At the start of gonadotrophin treatment, patients attended for a baseline assessment of the male, as well as the female partner. Male patients underwent clinical examination or testicular ultrasound, SA and blood tests for LH, FSH, Testosterone and Oestradiol. If the patient was on exogenous testosterone replacement, a wash-out period, from 1 to 14 weeks, was required before staring gonadotrophin treatment, to allow for a baseline assessment. While on gonadotrophin therapy, patients would undergo hormonal profile monitoring every three months, and repeat SA every three to six months.3 The target was to increase concentration of testosterone above the arbitrary cut-off level of 15 nmol/l and stimulate spermatogenesis.4 Patients with PH including Klinefelter syndrome, received 6 months of gonadotrophin treatment to stimulate testicular Leydig cells prior to microTESE.
Data collection
A service evaluation questionnaire was distributed to all men, covering overall service accessibility, hypogonadal symptoms while undergoing spermatogenesis induction, access to specialist examinations and understanding of the results. All questions were open-ended with a free text option to provide participants the opportunity to express their answers in detail. Related clinical information were retrieved from patients’ electronic records and the reproductive medicine unit laboratory.
Statistical analyses
All statistical analysis was performed using Graph Pad Prism 5. Quantitative data was assessed for normality using D’Agostino-Pearson normality test, followed by appropriate parametric (Unpaired t-test) or non-parametric (Mann Whitney U test) analysis. Group comparisons with respect to categorical variables were performed using Fisher’s-exact test or Chi-Squared test. All hypothesis testing was two-tailed; p<0.05 was considered statistically significant. Data are presented as either mean + standard error of mean (SEM) or median, interquartile range; IQR), as applicable.
Participant characteristics
Eighty-seven of 168 (52%) potential participants responded to a request to take part in the audit and service evaluation process. The remaining 81 men no longer attended the Male Fertility clinic, therefore unable to evaluate the service and unsuitable for audit inclusion.
Mean age of participants was 38 years, ranging from 21 to 63 years old (SD 9.6). They were of a multi-ethnic background; 38 (45%) were Caucasian, 11 (13%) were Black African or Caribbean and 35 (42%) were of other ethnic backgrounds. The most frequent diagnosis was CH, with multiple pituitary deficiencies in the context of prolactinomas, somatotropinomas or non-functioning macroadenomas, in 33 (39%) men (Figure 1).
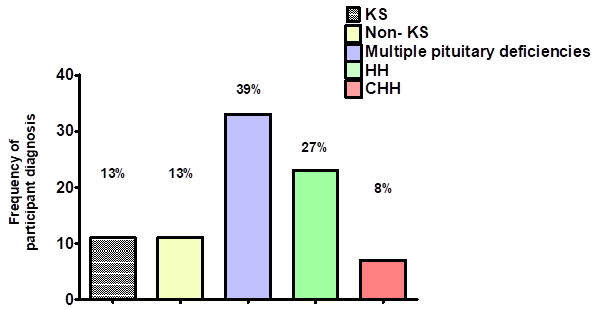
Figure 1 Frequency of diagnosis for men referred to the Fertility clinic for induction of spermatogenesis. KS; Klinefelter syndrome, Non-KS; primary testicular failure non associated with KS, HH; Hypogonadotrophic hypogonadism, CHH; Congenital hypogonadotrophic hypogonadism.
Access to the Fertility clinic
Participants were referred to the clinic from general endocrinology, paediatric endocrinology or urology services. Fifty-seven (68%) men felt that the waiting time for their first appointment was acceptable. Twenty-five (30%) had a long wait, between 6 to18 months before their first appointment, with a median wait of 12 months. From the current questionnaire it was not possible to ascertain the reason for increased waiting time before the first appointment.
Symptoms of low Testosterone on transition to gonadotrophins
Seventy-six (90%) men were on testosterone replacement before induction of spermatogenesis. Nineteen (23%) men reported being on gonadotrophin therapy before the start of the service evaluation period, and did not experience symptoms associated with low testosterone. Fifty-one (60%) men reported that the transition onto gonadotrophin therapy was not associated with any symptoms. Thirteen participants (15%) reported they were troubled with low libido and erectile dysfunction during gonadotrophin titration.
Testicular examination
Seventy-seven (92%) men had their testes examined and had a discussion with the health care professional regarding the significance of the findings, including whether the testes were appropriate size for their developmental stage. Fifteen out of the seventy-seven (19%) men had testicular ultrasound. Seven (8%) men did not have a testicular examination; one man declined testicular examination.
Semen analyses and outcomes of spermatogenesis induction
Men presented at the Fertility clinic with azoospemia or severe oligospermia (<5million/ml). A total of 58 out of 84 (68%) men attending clinic, successfully underwent spermatogenesis induction. Forty-nine (58%) participants had sperm seen in their SA ejaculate spontaneously and 8 (10%) men after microTESE (Figure 2).
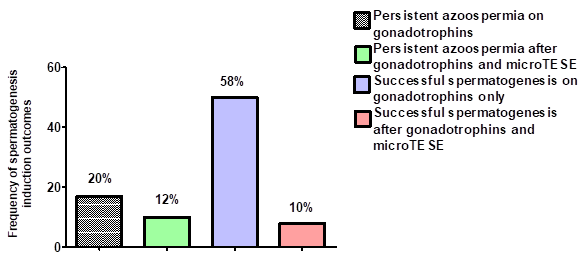
Figure 2 Frequency of spermatogenesis induction outcomes for men referred to the Fertility clinic. MicroTESE; Microdissection testicular sperm extraction.
Men with persistent azoospermia were more likely to have a diagnosis of PH (Odds ratio 22.5, 95%CI: 6.3 – 81.2, p<0.001) and smaller testicular size at baseline (Odds ratio 8.8, 95% CI: 2.6 – 30, p<0.001) compared to men with successful spermatogenesis. Only three out of twenty-two (14%) men with PH had successful spermatogenesis induction. Further subgroup analysis, showed that men with persistent azoospermia were more likely to have a diagnosis of KS (Odds ratio 38.5, 95% CI: 4.6 – 321,3, p<0.001) compared to men with successful spermatogenesis. Induction of spermatogenesis had a more favourable outcome in men with CH, as fifty-three out of sixty-two (86%) men had successful spermatogenesis outcome.
Understanding of the clinical investigations
Mean sperm concentration, total sperm count, total motility and morphology percentage for men, who had SA in our study, are summarised in Table 1. Seventy-one (85%) men were aware of their SA results but 13 (15%) said the results were unknown to them. Two men reported that their SA results were due to be discussed at later stage as they were not keen to discuss imminently. Some men, who were relatively early in the course of treatment, may have been unfamiliar with reviewing the SA results and their interpretation.
|
Parameters |
Sperm cryopreservation (n=10) |
Seeking fertility |
WHO criteria (2021) Lower reference limit with 95% CI |
|
|
No live birth (n=30) |
Live birth (n=17) |
|||
|
Sperm concentration (million/ml) |
29.9± 14.6 |
21.7±5 |
13.4± 8.8 |
>15 |
|
Total sperm count (million) |
36.9± 13.3 |
65.±16.7 |
26.9±11.2 |
>39 |
|
Total motility |
48.6± 8.2 |
43.1±5.9 |
33.3±5.1 |
>40% |
|
Total morphology |
2.3± 0.6 |
3.1±0.8 |
2.1±0.6 |
>4% |
Table 1 Semen analysis parameters according to intention to induce spermatogenesis with gonadotrophin therapy. Data presented as mean ± SEM. CI, confidence interval
Fertility outcomes
Thirteen men of 47 (28%) had partners, who conceived spontaneously and delivered healthy babies. Four men of 47 (9%) had a live birth after ART (Figure 3). Only 1 of 2 men with PH seeking fertility, had a partner with a positive pregnancy test, which resulted in a miscarriage. There were no live births in this particular cohort of men with PH. Live birth rate was higher in men with CH, with 17 of 45 (38%) men having a partner that successfully delivered a baby.
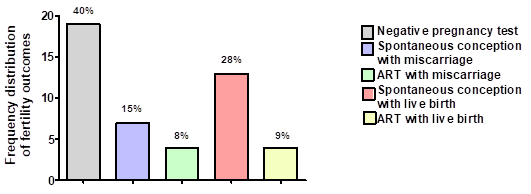
Figure 3 Outcomes for men seeking fertility and referred to the Fertility clinic for induction of spermatogenesis. ART; assisted reproduction treatment.
Comparison of endocrine parameters, clinical characteristic and SA of men with live birth to those without a live birth
Baseline endocrine parameters were similar between participants with live births and those who did not experience a live birth. That was expected, as only two men with PH and elevated gonadotrophins sought fertility, and the remaining forty-five men had CH, with similarly low gonadotrophins and comparable baseline testosterone levels on replacement, between the two groups (Table 2).
|
Parameter (reference range) |
Live birth (n=17) |
No live birth (n=30) |
|
LH (1.7 -8.6 iu/L) |
0.6 ± 0.2 |
1.5 ± 0.5 |
|
FSH (1.5 – 12.4 iu/L) |
4.6 ± 1.7 |
2.3 ± 0.8 |
|
Estradiol (<192 pmol/L) |
172.2 ± 4.2 |
151.8 ± 24 |
|
Testosterone (7.6 – 31.4 nmol/L) |
16.4 ± 2.5 |
15.7 ± 1.7 |
Table 2 Baseline endocrine parameters of participants seeking fertility. Data presented as mean ± SEM. *** p<0.001. LH, luteinizing hormone; FSH, follicle stimulating hormone
Clinical characteristics of men, whose partners had a live birth, were similar to men without a live birth. Interestingly, men without a live birth trended towards having partners with subfertility (pelvic inflammatory disease, endometriosis or polycystic ovarian syndrome) though this did not reach statistically significance (Odds ratio 6.9, 95%CI: 0.8 – 59.9, p=0.07) (Table 3). It is interesting that only 6 out of 17 (35%) men with partners above 35 years had a live birth, compared to 11 out of 17 (65%) men with partners below 35 years and a live birth.
|
Parameter |
Live births (n=17) |
No live births (n=30) |
|
Age (years) |
38.4 ± 1.9 |
42.2 ± 1.6 |
|
BMI (kg/m2) |
28 ± 1.4 |
37.1 ± 7.2 |
|
Smoker (%) |
1 (6%) |
2 (7%) |
|
Alcohol Intake >10 units/week (%) |
1 (6%) |
4 (13%) |
|
Partner older than 35 years old (%) |
6 (35%) |
13 (43%) |
|
Partner with subfertility (%) |
1 (6%) |
9 (30%) |
Table 3 Clinical characteristics of participants seeking fertility. Data for age and body mass index (BMI) presented as mean ± SEM
The timespan on gonadotrophin therapy was significantly lower in men without a live birth, compared to men with live birth (mean timespan in months: 24 ± 3, no live birth; 38 ± 5, live birth, p<0.05). It is not possible from this survey to explain the difference in gonadotrophin treatment timespan, between the two groups. Finally, sperm concentration, total sperm count, total motility and morphology percentage were non-significantly different between men with or without live births (Figure 4).
Male factors contribute to almost half of the cases of infertility. Despite advances made in infertility management, treatment options for male infertility remain limited. Gonadotrophin therapy has been offered by our clinic to men with central hypogonadism and primary hypogonadism, characterised by azoospermia. This study reported on spermatogenesis and fertility outcomes, as well as the male patients’ perspective on their fertility management. Fifty-seven (68%) men overall had successfully undergone spermatogenesis induction and 17 (36%) out of the 47 who proceeded with fertility, had partners who went on to have live births.
Evaluating the experience of men attending fertility clinics can provide important feedback when considering modifications to the service. Men attending this clinic had diverse ethnic backgrounds, with a mean age of 38 years old and were predominantly diagnosed with CH in 73% of cases. Participants mainly reported ease in obtaining a first appointment, but a minority (30%) of men had a median wait of 12 months before an initial assessment. The reasons for delayed assessment in the clinic was not identified by this study. NICE guidelines do comment on the need for effective referral pathways, opportunities should be sought to improve awareness and facilitate prompt patient referrals.16
Nine in ten men referred for induction of spermatogenesis were treated with testosterone, before their first appointment. Only a small proportion of men (15%) reported symptoms of low testosterone, whilst transitioning from testosterone replacement to gonadotrophin injections. Liu et al. showed that men not previously on testosterone are better responders to spermatogenesis induction with gonadotrophins.5 Exogenous testosterone treatment in young males with hypogonadism can promote premature maturation of Sertoli cells, that could negatively impact future fertility.6,7 Early gonadotrophin therapy might be advocated in preference to testosterone replacement for young hypogonadal men. However, a prospective study of 60 male adolescents, with CH on gonadotrophin therapy for induction of spermatogenesis, showed similar spermatogenesis outcomes between those exposed to testosterone for puberty induction and those not previously exposed to testosterone.8 Gonadotrophin therapy for pubertal induction can be difficult to administer, costly and may be slow to induce pubertal changes in a cohort of young men experiencing the psychological strain of keeping up with the development in their peers. In general, gonadotrophin therapy is preferable when considering the long time that fertility induction may require.
Testicular size is a significant factor when predicting outcomes of spermatogenesis induction, with small testicular size commonly reflecting poorer spermatogenesis potential.6 Testicular examination before the commencement of gonadotrophin treatment remains essential. However, men can find testicular examinations embarrassing and uncomfortable, with 8% of men in our study not having a testicular examination at baseline, and one man refusing examination. The routine use of testicular ultrasound during spermatogenesis induction is less common compared to testicular examination via orchidometer. Testicular examination via orchidometer is rapid and simple, but there can be considerable variation between different assessors. In contrast, testicular ultrasound can be precise and helpful to identify cases of obstructive azoospermia, when considering the possibility of an obstructive cause for infertility.9
The percentage of successful spermatogenesis was expectedly higher at 86%, in men with CH, compared to the 14% success rate in men with PH. Overall, more than two thirds of men attending clinic achieved spermatogenesis. Rates of successful spermatogenesis in both CH and PH groups, were similar to previously reported rates in other centres, according to published literature.5,14 Eighty-five per cent of men with CH had successful spermatogenesis outcome and their success rate was equal to the rate recently reported by Ortac et al.10 Only 14% of men with PH had successful spermatogenesis induction and this rate is similar to the previously reported 11%-15% rates in men with PH, either after gonadotrophin therapy alone or gonadotrophin therapy followed by micro-TESE.11,12
Fifteen percent of men reported not being made aware of their SA results. This percentage reiterates the importance of ongoing dialogue to keep patients informed of their treatment duration and their specific treatment targets. Discussions about next steps and education is essential to increase concordance with treatment, which is critical given the significant costs of therapy and the potentially prolonged nature of therapy.
Live birth rates in our study were 38% for men with CH over a mean gonadotrophin treatment period of 38 months. Moreover, live births rate was similar to the 40% rate reported in a retrospective study, involving men with a mean age of 28 years old, who received gonadotrophin therapy over a mean period of 26 months.13 Most recent studies reported higher live birth rates between 56% to 65%.14,15 Several reasons might be postulated to account for higher live birth rates in recently published series, when compared to our study. For example, Liu et al. offered gonadotrophin therapy to men with CH, with mean age of 22 years old and no previous testosterone use. Similarly, men from the study published by Yilmazel et al. were younger compared to men seeking fertility in our study, with a mean age of 20 years old.14 The mean age was 40 years old in our study, which was older than the quoted studies and this may reflect reduced reproductive potential from potentially greater proportion of female partners above the age of 35.
It is recognised that men with PH and spermatogenic failure with no reversible cause, have very limited response to fertility treatments. Expectedly, there were no live births in men with PH. Although this observation is not in agreement with the 52% live birth rate reported by Reifsnyder et al, the very small number of men with PH, seeking fertility in this study, does not allow drawing further conclusions.16
Our study is the first to compare the clinical, biochemical and seminal characteristics between men with live births versus those without a live birth, undergoing induction of spermatogenesis with gonadotrophin therapy. Although men from the two groups were not significant different in terms of their hormonal profile, BMI or alcohol intake, 30% of couples without a live birth included female partners with underlying subfertility.17 Men that did not have live births trended towards partners with diagnoses of pelvic inflammatory disease, endometriosis or polycystic ovarian syndrome. Also, more than a third of men (19/47) had partners above 35 years of age; with the percentage of men having a partner below 35 years and live birth (65%) being double, compared to the percentage of men having a partner above 35 years and a live birth (35%). It has been shown that the woman’s age is a prognostic factor for pregnancy, with pregnancy rates declining after the age of 30 years, especially among those women seeking assisted reproduction.18 Seminal sperm parameters were not statically different between groups, which lends weight to other factors, other than men’s reproductive status, being important.
Limitations
This was a questionnaire-based study capturing patient’s perspective on their fertility journey with respect to spermatogenesis induction and fertility outcomes in a Fertility Clinic. The response rate to our study was 50%. It has been reported that men find it difficult to participate in studies investigating their reproductive status19 and this was evident from our study’s response rate. Therefore, outcomes based on the current responses may not be representative and might introduce bias with difference between the responders and non-responders. We used a tailored questionnaire for the purpose of the study, but this was not an externally validated questionnaire. Practice across fertility clinics varies and data were presented from a single UK centre, therefore may not be representative of practice and outcomes in other centres.
Men attending our Fertility clinic were promptly referred and seen for induction of spermatogenesis. Most men were on prior testosterone therapy and transition from testosterone replacement to gonadotrophins was generally uneventful, without the development of frequent hypogonadal symptoms. Testicular examination is an essential part of the initial assessment. Routine testicular ultrasound at baseline may be a useful aide, as men attending fertility clinics are reluctant to undergo testicular examination. Spermatogenesis induction was effective, particularly for the CH cohort. Ensuring male subjects are well informed about duration and targets of therapy, potential outcomes and prognostic factors, along with feedback about response to therapy, is likely to improve patient experience and overall outcomes.
None.
Ethical approval
This study was approved by the local Clinical Governance Committee, University College Hospital London, on 15th December 2021, to conduct a service evaluation study for the Fertility clinic.
Consent
Written informed consent was waived by the local Clinical Governance Committee, University College Hospital London, as this was a study to audit practice and evaluate the service. Data are anonymised.
Authors’ contributions
AD, GC and US conceptualised the study. PP and AW contributed equally. AD, PP, AW, EW and FA were responsible for accessing and maintaining data. All authors reviewed and edited the article.
None.
The authors declare that there are no conflicts of interest regarding the publication of this paper.

©2024 Dimakopoulou, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.