eISSN: 2377-4304


Research Article Volume 12 Issue 2
1Servicio de Obstetricia y Ginecología, Hospital Clínico Universitario de Valladolid, Gerencia Regional de Salud de Castilla y León (SACYL), Spain
2Departamento de Pediatría e Inmunología, Obstetricia y Ginecología, Nutrición y Bromatología, Psiquiatría e Historia de la Ciencia, Facultad de Medicina, Universidad de Valladolid, Spain
Correspondence: Dakota Viruega-Cuaresma, Dakota Viruega-Cuaresma, Servicio de Obstetricia y Ginecología, Hospital Clínico Universitario de Valladolid, Gerencia Regional de Salud de Castilla y León (SACYL), España, Avenida Ramón y Cajal 3, 47005 Valladolid, España, Spain, Tel +34667219280
Received: February 22, 2021 | Published: March 15, 2021
Citation: Viruega-Cuaresma D, De-Miguel-Manso S, García-García E, et al. Six-year follow-up in patients with urinary stress incontinence treated with Altis® single-incision sling: a prospective single-center study. Obstet Gynecol Int J. 2021;12(2):72-76. DOI: 10.15406/ogij.2021.12.00554
Objetives: Single incision slings are the latest generation of suburethral bands that seek to minimize morbidity and major complications of transobturator bands. In short and medium term, their results in terms of success and safety are similar to transobturator and retropubic bands. Nevertheless, there is little data on their long-term outcomes. Our objective was to evaluate safety and efficacy of Altis® mini-sling during the short, medium and long-term follow-up.
Methods: Prospective observational study in 67 women who underwent surgery with Altis® for a period of 6 years (2013-2019). The main variables evaluated were: total continence, objective cure and subjective cure (satisfaction). The secondary variables studied were: complications and number of pads used per day after surgery. Statistics: Student t-test or U-Mann-Whitney for quantitative variables, Chi-Square for categorical variables.
Results: Objective cure rates were: 96.77%, 87.04%, 87.50%, 85.71%, 76.92% and 50% (from 1 to 6years), and total continence: 85.48%, 72.22%, 64.88%, 50%, 46.15% and 50% (from 1 to 6years). Degree of satisfaction was: 9, 8.2, 8.3, 7.7, 7.6 and 6.5 points (from 1 to 6years).
30 patients (44.78%) had some type of complication and the most common were: “de novo” urinary urgency (20.90%), recurrence of stress urinary incontinence (14.93%) and pain (5.97%).
Conclusion: Altis® presents high rates of objective and subjective continence in short and medium-term (1-5years), during 6-year follow-up. It is safe and does not associate severe complications. We found a high percentage of “de novo” urgency and recurrence of stress urinary incontinence, especially after the fifth year.
Keywords: altis®, six-year follow-up, urinary stress incontinence
There is little data on their medium and long-term outcomes. Altis® presents high rates of objective and subjective continence in short and medium-term, during 6-year follow-up.
SUI, stress urinary incontinence; SIS, single incision sling; MUI, mix urinary incontinence; POP, pelvic organ prolapse; BMI, age and body mass index; UDS, urodynamic study
Stress urinary incontinence (SUI) is defined according to the ICS (International Continence Society) terminology standardization, such as involuntary loss of urine after physical exertion (sport, activity) or after coughing or sneezing.1 It is estimated that its pre-valence in the postmenopausal female population ranges between 10 and 40%, representing the most common form of incontinence.2,3 These symptoms affect physically and psychologically in patient´s quality of life.
Treatment of SUI depends on the degree of severity.4 In general, therapy is initiated by indicating lifestyle modifications and rehabilitation of the pelvic floor musculature.4,5 However, in moderate and severe cases, surgery is positioned as the treatment of choice for the majority of patients.6
Introduction of synthetic suburethral polypropylene bands represents one of the most significant surgical advances in recent decades, replacing retropubic colposuspension (the traditional SUI´s surgical approach),7 positioning itself as the “gold standard” for the surgical treatment of SUI.8
The minimally invasive single incision sling (SIS) or mini-slings are the latest generation of suburethral bands, and they were born in 2006. Mini-slings were designed with the aim of minimizing the morbidity and major complications of transobturator bands.9
The published studies evaluating SIS´s efficacy in the short and medium term are encouraging, reflecting success and safety rates similar to transobturator and retropubic bands.10–13 Nevertheless, it is not yet possible to take a firm stance on this new generation of suburethral bands.
Altis® single-incision sling (Coloplast Corp., Minneapolis, MN, USA) has the property of providing an adjustable tension to the implant. It was approved by the United Stated Food and Drug Administration (FDA) in 2012 and is indicated for the treatment of female SUI secondary to urethral hypermobility and/or intrinsic sphincter deficiency.
The limited data published is mostly short-term follow-up. The objective of our study was to evaluate the safety and efficacy of the Altis® mini-sling for SUI during the short, medium and long-term follow-up.
A prospective observational study was conducted in 67 women with SUI who underwent surgery with an Altis® mini-sling for a period of 6years (2013-2019).
All the patients were operated on at the Universitary Clinic Hospital of Valladolid and evaluated at the Pelvic Floor Unit. The surgeries were performed by two gynecologists as an outpatient procedure, following the same surgical technique, under sedation and local anesthetic infiltration. They were discharged the same day, without bladder catheterization and after verifying a postvoid residue of less than 100 ml. All patients signed the informed consent before the procedure.
The inclusion criteria were that the patient presented SUI, associated or not with mixed urinary incontinence (MUI), with a predominance of stress urinary incontinence symptoms, a positive cough stress test with urethral hypermobility, and failed or unwanted previous conservative treatment for SUI.
Exclusion criteria were incontinence of neurogenic cause or suspected intrinsic urethral sphincter deficiency, the need for concomitant surgery associated with pelvic organ prolapse (POP), voiding dysfunction, and previous surgical treatment of SUI. Age and body mass index (BMI) did not limit inclusion in this study.
The patients were evaluated with respect to other variables such as anthropometrics (age and BMI), obstetric history (parity, vaginal delivery with fetus >4kg, instrumented delivery with forceps), comorbidity (arterial hypertension, respiratory, neurological or psychiatric pathology), previous gynecological surgery, type of urinary incontinence (SUI, MUI), severity of SUI (grade I, II, III responding to minimal, moderate or great efforts, respectively), number of pads used, before and after surgery, and complications.
The preoperative study included: detailed medical history, exclusion of a urinary tract infection, physical examination and evaluation of incontinence (cough test, Bonney test), as well as a gynecological ultrasound to objectify the existence of urethral hypermobility and rule out pathological postvoid residual.
If the patient had MUI, the urgency component was first treated with behavioral and pharmacological interventions for at least 6 months.
Urodynamic studies (UDS) were only performed when we suspect intrinsic urethral sphincter deficiency as a potential cause of SUI and in complex urinary incontinence.
During the first 2years, the follow-up was carried out in the Pelvic Floor Unit consultation by physical examination, cough test, measurement of residual urine and visual satisfaction scale. Afterwards, annual follow-up was stablished, by telephone interview, carrying out an exhaustive anamnesis on symptoms related to SUI, urgency and urge urinary incontinence.
Patient satisfaction was established by themselves, on a scale between 0 and 10.
The main variables evaluated were:
Statistical analyses
The Kolmogorov-Smirnov test was applied for quantitative variables to determine the type of distribution. The Student t-test was used to study quantitative variables with a normal distribution, and the U-Mann-Whitney test was used otherwise. The Chi-square test with Yates correction was used to study categorical variables. In all cases, less than 0.05 was the value taken as statistically significant. SSPS v. 23 was the statistic software used.
Ethical approval
Once the study was approved by the hospital's research committee (Code: FO-P07-12, approval date: 03-18-2016 and start date of the study: 02-13-2015) (Appendice-1), after giving our patients verbal informed consent by telephone, we collected the data from the clinical history of the first two years after surgery, and we completed by telephone follow-up until the sixth year.
We included 67 patients operated with Altis® mini-sling, between 2013 and 2019. The demographic, clinical and urodynamic characteristics of the population are shown in Table 1. The mean age was 55.8±12.1years and the BMI was 26.7±4.3kg/m2. Regarding type of incontinence, 46.27% presented MUI with a predominance of stress incontinence and 53.73% pure SUI (92.54% Grade II and 7.46% Grade III). An urodynamic study was carried out in 18 patients (28.87%).
|
Variable |
Valor |
|
Age (mean, SD) |
55.8±12.1 |
|
BMI (kg/m) (mean, SD) |
26.7±4.3 |
|
Tobacco (%) |
17.39 (12/67) |
|
Physical activity (%) |
42.72 (31/67) |
|
Vaginal parity ≥2 (%) |
95.52 (55/67) |
|
Instrumental delivery with forceps ≥1 (%) |
8.96 (6/49) |
|
Fetal macrosomía ≥1 (%) |
19.40 (13/52) |
|
Medical diseases (%) |
|
|
Arterial hypertension |
29.85 (20/67) |
|
Respiratory Pathology |
8.96 (6/67) |
|
Psychiatric illness |
29.85 (20/67) |
|
Neurological Pathology |
2.99 (2/67) |
|
Urinary Incotinence Type (%) |
|
|
SUI |
53.73 (36/67) |
|
MUI |
46.27 (31/67) |
|
Degree of SUI (%) |
|
|
Grade II |
92.54 (62/67) |
|
Grade III |
7.46 (5/67) |
|
Number of pads prior to surgery (mean±SD) |
3.52±1.8 |
|
Previous Urodynamic Study (%) |
28.87 (18/67) |
|
Surgical time (minutes) (mean±SD) |
27.4±10.8 |
Table 1 Demographic and Total continence rate and objective cure (cough test) of patients with Altis® during the 6-year follow-up
BMI, age and body mass index; SUI, stress urinary incontinence; MUI, mix urinary incontinence
All patients underwent successful Altis® placement and the intervention time was 27.4±10.8min. Patients who completed six year follow-up are reflected in Table 2.
|
Year of follow-up |
Number of patients operated with Altis® who completed the follow-up of the total |
|
1 year |
62 (67) |
|
2 years |
54 (67) |
|
3 years |
48 (67) |
|
4 years |
28 (67) |
|
5 years |
16 (67) |
|
6 years |
2 (67) |
Table 2 Number of patients operated with Altis® who completed the follow-up each year during the study period
Concerning the main variables (Figure 1), the total continence rate and objective cure at 12 months were 85.48% and 96.77%, respectively.
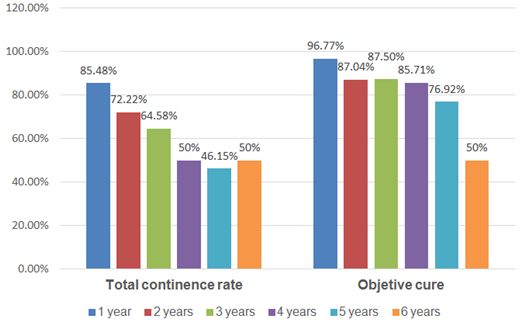
Figure 1 Total continence rate and objective cure (cough test) of patients with Altis® during the 6-year follow-up.
During the medium and long-term follow-up, the objective cure rates were: 87.04% (2years), 87.50% (3years), 85.71% (4years), 76.92% (5years) and 50% (6years), and total continence: 72.22% (2years), 64.88% (3years), 50% (4years), 46.15% (5years) and 50% (6years).
The degree of satisfaction (Figure 2) over the years was: 9 points (12 months), 8.2 (2years), 8.3 (3years), 7.7 (4years), 7.6 (5years) and 6.5 (6years). The number of pads used after surgery is reflected in Figure 2.
Regarding safety, 30 patients (44.78%) had some type of complication that we classified as immediate (first 7 days), intermediate (7-30 days) and late (>30 days), which are shown in Table 3. All of them were grade I complications according to the Clavien-Dindo classification,14 except for one patient who required Altis® mesh section due to acute urine retention.
|
|
Complication |
N (%) |
|
Immediate (8.96%) |
Urinary tract infection |
2 (2.99) |
|
Urinary retention |
1 (1.49) |
|
|
Vaginal Perforation |
3 (4.48) |
|
|
Intermediate (2.99%) |
Urinary tract infection |
2 (2.99) |
|
Late (32.84%) |
De novo urgency |
14 (20.9) |
|
SUI relapse |
10 (14.93) |
|
|
Pain |
4 (5.97) |
|
|
Vaginal mesh extrusion |
2 (2.99) |
Table 3 Distribution of postoperative complications with Altis® during the 6-year follow-up
SUI, stress urinary incontinence
The most common complications were: “de novo” urinary urgency (20.90%), recurrence of SUI (14.93%) and pain (5.97%).
In the last few years, there have been several published studies evaluating the short-term efficacy and safety of the Altis® mini-sling with promising results. However, there are few studies to assess them in a long-term. It´s difficult to advance the knowledge of mini-slings for several reasons, mainly due to the heterogeneity of the studies, from a methodological point of view, the type of population included and the duration of follow-up.
Most of the scientific publications are retrospective observational studies,13,15 and prospective single-center16,17 or multicenter.18 Our work is a prospective observational single-center study with a 6-year follow-up. The literature reviewed reports follow-ups at 1year,13,16,19,20 2years17,18 and 3years.15
Our work includes women with a history of pure SUI and MUI with a predominance of stress incontinence, a positive cough test in consultation with urethral hypermobility, and failed or unwanted previous conservative treatment.
Exclusion criteria were incontinence of neurogenic cause, suspect intrinsic urethral sphincter deficiency, the need for concomitant surgery associated with POP, voiding dysfunction, and previous surgical treatment of SUI. These data are consistent with the criteria used by other authors.16,17,20
Overall, the objective efficacy of Altis® was high and sustained. The objective cure rate at 12 months was 96.77%, which is higher from that previously reported by other authors, such as Dias16 who reports an objective cure of 90.2% and Tao Jiang20 of 91.2%.
At 24 months, our objective cure decreased to 87.04%, as described in other studies. Morán17 establishes a cure of 82.7% or Kocjancic18 of 87.9%. These differences may be due to the criteria used to assess objective cure.
We used the cough test with a full bladder (250-300ml) checked by ultrasound; however, other authors such as Kocjancic18 used a 50% or more reduction in the weight of the pads. At medium-term follow-up, the objective continence rate remains stable, being 87.50% at 3 years and 85.71% at 4years.
However, that is not the case when we speak of total continence, understanding this as the absence of urine leakage (due to urgency or effort). At 12 months, a total continence of 85.48% is reported; with a clear tendency to get worse over the years: 72.22% (2years), 64.88% (3years) and 50% (4years). This is surely connected to the appearance of urge urinary incontinence with aging, or worsening of the urgency in MUI, rather than recurrence of SUI (most of them had a negative cough test).
At long term, the total continence rate shows a tendency to decrease: 46.15% (5years) and 50% (6years). This long-term worsening trend is more evident in objective cure: 76.92% (5years) and 50% (6years).
The patients presented a high degree of satisfaction, obtaining a mean of 9 points on the visual satisfaction scale in the short-term follow-up. Furthermore, no significant decrease in patient satisfaction was observed in the medium term, remaining stable between 8.2 and 7.6 points (2-5years). Even at 6 years, the average score of the 2 patients who completed the 6year- follow up, was 6.5. These results are similar to those reported by Morán17 with a mean of 9.5 points at 22 months. Dias16 and Henry,19 used the ICIQ-SF questionnaire. On the other hand, the authors Kocjancic18 and D'Alessandro15 used the PGI-I questionnaire to analyze this variable, obtaining very high degree of satisfaction in 90.4% (2years) and 87.23% (3years) respectively. The quantification of subjective cure with different scores and questionnaires makes it difficult to compare the results between studies.
Our global complication rate was 44.79%, higher than the 21.8% described by Morán,17 who only considers as complications: vaginal erosion, acute urine retention, voiding dysfunction and pain. Morán does not include urgency or recurrence, which may justify this disparity of percentages.
There were no severe complications. Late complications (32.84%) were the most frequent, followed by immediate (8.96%) and intermediate (2.99%). There were 2 mesh extrusions (2.99%), same proportion as for other authors: 1.92% (16), 2.12% (15), 3.50% (18) and 2.94% (20). In contrast, Morán17 does not report any extrusion in his series.
Perhaps, the most striking feature is the percentage of women with “de novo” urgency (20.90%), higher than the figures described by Moran17 (8.1%), D’Alessandro15 (6.38%) and Dias16 (5.9%).
A review of 10 articles including more than 2800 patients21 that evaluated risk factors and management of overactive bladder after SUI surgery, found several risk factors for “de novo” urgency: obesity, advanced age, poor bladder capacity, high pressure detrusor or history of SUI surgery. Advanced age and previous MUI seem to be the most consistent risk factors. We did not find differences with other authors in relation to age. In our sample the mean age was 55.8years, similar to that described in other publications: 53.2years,16 56.9years15 and 59.7years.17 Other factors that could influence would be: the location of the band in relation to the urethral neck, the proximity of the band to the urethral wall or the thickness of the urethra itself. We believe that careful patient selection could achieve better results.
Finally, our frequency of SUI´s recurrence is not negligible, 14.93%. Given the sharp drop in the objective continence rate from the fifth year, maybe a recu-rrence is more likely thereafter.
Limitations of this study
None.
Dakota Viruega-Cuaresma, PhD: Manuscript writing, Data Collection.
Sonia De-Miguel-Manso, PhD: Project development, Manuscript writing, Data Collection.
Elena García-García, PhD: Data Collection, Manuscript writing.
Carmen E. Badillo-Bercebal, PhD: Data Collection.
Julio A. Gobernado-Tejedor, PhD, MD: Project development, Statistical analysis.
Marta Pérez-Febles, PhD: Data Collection.
No funding was obtained for the implementation of this study.
The authors declare that they have no conflict of interest.

©2021 Viruega-Cuaresma, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.