eISSN: 2377-4304


Review Article Volume 12 Issue 3
Obstetrician gynecologist, Perinatologist (CLAP PAHO/WHO), Institute of Public Health, Spain
Correspondence: Manuel Sánchez-Seiz, Obstetrician gynecologist, Perinatologist (CLAP PAHO/WHO), Ultrasound Unit, Madrid + Health, Institute of Public Health, Madrid, Spain
Received: April 22, 2021 | Published: May 21, 2021
Citation: Sánchez-Seiz M. Screening for aneuploidies in twin gestation. Obstet Gynecol Int J. 2021;12(3):160-178. DOI: 10.15406/ogij.2021.12.00571
The prenatal screening for fetal chromosomal abnormalities (FCA) was initially based on epidemiological data provided by pregnancy, with the greatest weight being maternal age compared to those provided by family history or clinical history.
Advanced maternal age was the first criterion for screening the risk of Down syndrome in the general population and was introduced in the early 1970s when the determination of the fetal karyotype became possible.1,2
It was known that the prevalence of SCD increased directly in relation to maternal age and decreased inversely with gestational age and,3 of the high fetal mortality found in these pregnancies.4 And it was considered that approximately one in 500 pregnant women would be a subsidiary of having a fetus with SCD at term. Of these fetuses only 30% will be born to mothers over 35years of age.5
Thus, the greatest number of newborns (NBs) carrying some type of SCD will come from mothers under 35 years of age.
Thus, this screening model based on maternal age is estimated to have a very low sensitivity, less than 30%, with a false positive rate of 10%.6 The arbitrary decision to give the maternal age of 35 as a cut-off point evidently it was conditioned by the limitation of economic resources.7 It is estimated that in Spain in the eighties of the last century, only 5% of the population of pregnant women was covered, which corresponded to pregnant women over 35 years of age. If the fetal karyotype were performed in all these pregnancies by invasive methods (amniocentesis, chorionic villi), only 25-30% of all FCAs would be identified with a loss rate associated with the procedure of approximately 1%.8
Since then, two factors have modified the selection criteria: the request for prenatal diagnostic methods by women under 35 years of age and the increasing number of women who become pregnant over the age of 35.9
Currently it is estimated that women older than 35 years are 7-10% of all pregnant women. If amniocentesis were offered only to them, 35% of all FCAs would be identified, but the number of invasive procedures would double (7-10%) and, consequently, the losses related to them.10
Since in 1984,11 found a direct relationship between decreased alpha-fetoprotein (AFP) values in maternal blood and the presence of ACF, a whole host of substances produced during pregnancy and detectable in maternal blood have been detected, which together with the technical development of ultrasound and the finding of ultrasound markers related to ACF, have opened the door to the screening of fetal aneupliodies by non-invasive methods, aimed at the general population of pregnant women.
The most used biochemical markers can be seen in Figure 1 and the most effective screening strategy in the first trismester of gestation is undoubtedly the combined test Figure 2.
Has recently published a clinical practice guide for the screening of fetal chromosomal abnormalities.12 Among the recommendations based on evidence, with scientific consistency (level III), it stands out that:
Table 1 summarizes the main screening strategies and detection rates for Down syndrome for a positive screening rate of 5%.
First trimester |
Second trimester |
First + second quarter |
Detection rate (%) |
TN |
64-70 |
||
TN PAPP-A + hCG total or liter |
82-87 |
||
Triple Screen (AFP + hCG + uE3) |
69 |
||
Quadruple Screening (AFP + hCG + uE3 + InhibinA) |
81 |
||
Integrated: |
94-96 |
||
Quarter one: TN PAPP-A |
|||
Second quarter: Quadruple Screening |
|||
Integrated (biochemical markers only): |
85-88 |
||
Quarter one: TN PAPP-A |
|||
Second quarter: Quadruple Screening |
|||
Progressive sequential: |
95 |
||
If the result is positive in the first trimester, a direct diagnostic test is offered. |
|||
If negative, second trimester screening is offered. |
|||
Final risk contemplates the results of both quarters. |
|||
Progressive sequential: |
88-94 |
||
If the result is positive in the first trimester, a direct diagnostic test is offered. |
|||
If it is negative, no further tests are carried out. |
|||
If the result is intermediate, second trimester screening is offered. |
|||
Final risk contemplates the results of both quarters. |
|||
Table 1 Screening strategies. Detection rate (for 5% positive results). Taken from ACOG Practice Bulletin 2007
Performing an early screening offers other added benefits. I know encourages the performance of earlier ultrasounds, with the possibility of expand to other indirect markers of chromosomal diseases other than the TN. It facilitates the detection of morphological anomalies already in phase embryo and early positive results make 6 option of offering chorionic biopsy as first trial option invasive.13
Currently, the possibility of introducing new biochemical markers is being studied, such as: invasive trophoblastic antigen (ITA), eosinophilic basic protein (proMBP), metalloprotease (ADAM12), as well as new ultrasound markers: nasal bone hypoplasia, Doppler ultrasound ductus venosus and the length of the ear among others; with the aim of improving detection rates and reducing false positives in prenatal screening for congenital anomalies.14
The risk of Down syndrome for pregnancy has been empirically validated only one estimated by using first trimester biochemical markers PAPP-A and βHCG and TN.15
The mean±standard deviation of maternal age at delivery in the study population was 31.02±5.36 years, including 3,456 (23%) pregnant women over 35 years of age. Table 2 shows the observed prevalence and the mean estimated post-test risk in each of the risk groups into which the 15,009 screened pregnant women have been divided.
Predicted risk in childbirth |
Number of cases of Down syndrome observed (attached) to |
Number of cases of unaffected Pregnant womenb |
Observed prevalenceb (1 in [a + b] / a) |
|
Riogo staple |
Half |
|||
1 in 5 or greater |
I in 3 |
8 |
16 |
I in 3 |
1 in 6 to 1 in 10 |
1 in 8 |
7 |
32 |
1 in 5.57 |
1 in II 2 I in 20 |
I in 15 |
two |
57 |
1 in 29.5 |
1 in 21 to I in 11110 |
1 in 60 |
10 |
251 |
1 in 26.1 |
1 in 101 to 1 in 300 |
1 in 203 |
6 |
550 |
1 in 92.6 |
1 in 301 to 1 in 1,000 |
1 in 621 |
4 (5.71) |
1,833 |
1 in 330 |
<I in 1,000 |
1 in 8,817 |
2 12.85) |
12,181 |
1 in 4,275 |
All pregnant women |
1 in 7,256 |
39 (41.56) |
14.97 |
1 in 361.2 |
Table 2 Predicted mean risk and observed prevalence of Down syndrome, based on the number of pregnant women. Affected and Unaffected in Each Risk Group Predicted Using First Trimester Combined Fetaltest and Screening
aIn the low-risk group, the number in parentheses represents the number of observed cases increased by 31%, to take into account the spontaneous intrauterine lethality of the cases of Down syndrome between the first trimester and the patron
bThe prevalence has been calculated using the attached number of observed cases
The detection rate for DS was 82.05% (32/39) (95% confidence interval [CI], 70% -94%), for a false positive rate of the 5.36%. The observed prevalence of Down Syndrome (DS) in the first trimester was 1 in 361. Figure 3 graphically represents the close correspondence between the predicted and observed prevalence across the range of possible risks. This correspondence is mathematically expressed by a correlation coefficient of 0.999967 (p <0.0001).15

Figure 3 Predicted Mean Risk and Observed Prevalence of Down Syndrome Using First Trimester Fetaltest and Commodified Screening. The diagonal line represents the perfect agreement between the two variables.
Although it would be ideal to have population parameters (mean±standard deviation and correlations of the markers in the population of affected and unaffected pregnant women, for each of the markers used) extracted from the screened population itself, at present only these are available from publications of various research groups. For this reason, all the computer systems for calculating the prenatal risk of DS currently available in our environment, including Fetaltest, are based on algorithms dependent on population data obtained in highly qualified research centers, in circumstances different from the usual clinical practice of our country, and from samples of pregnant women with different characteristics from the population of pregnant women in our environment (eg, maternal). However, at the present time, we do not know of any computer system or method for calculating the risk of DS that has been validated in our setting and with our population of pregnant women. Although the present study includes a small proportion of pregnant women residing in Latin American countries (6.3%), most of the pregnant women screened reside in Spain (and more than 80% were recruited from the Spanish National Health System, in screening programs performed on the general population), so the study essentially represents the usual circumstances of the clinical environment in Spain.
This study confirms that the risks estimated by Fetaltest are accurate and highly consistent with the observed prevalence, as presented in Figure 3, with a high correlation between both parameters, r=0.999967, which is comparable to that published by other groups. 0.988 and 0.9995. This confirmation gives full validity in our environment and clinical circumstances to the logistics system (including training of sonographers, calculation system and quality control) that Fetaltest uses for the combined screening of the first trimester, which is important for the tranquility of the professionals who use or are willing to use Fetaltest, or are involved in the care of pregnant women screened with this prenatal screening tool.15
The multiple pregnancy rate is experiencing a continuous increase in developed countries. Without a doubt, the full incorporation of women into the world of work and the increasing complexity of the time devoted to study and professional training are determining factors in the delay of the age in which the woman looks for the first gestation. On the other hand, the increase in single-parent families or the search for gestation by homosexual couples means that assisted reproductive techniques (ART) have had a significant rebound and are no longer limited only to the world of infertility. It is estimated by various authors that the twin gestation rate is in the order of 33.2 per thousand.16
In Spain, although the Gross Birth Rate has been decreasing (Figure 4), the maternal age of arrival at gestation has been progressively increasing (Figure 5).
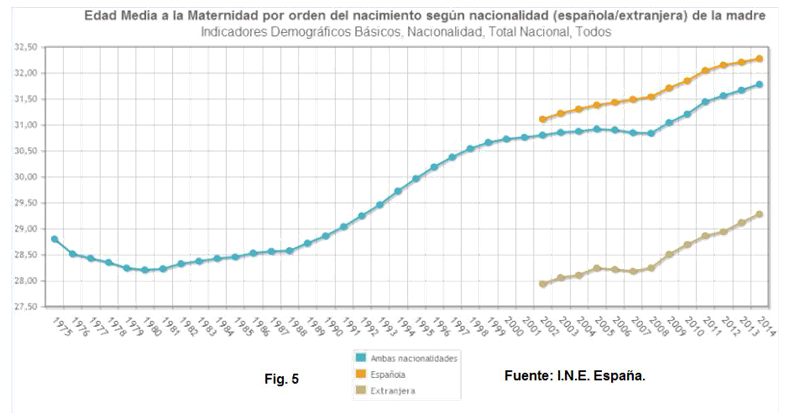
Figure 5 Average age at maternity in order of birth according to nationality (Spanish / foreign) of the mother.
And as we see in Figure 5, it is very striking that even though the age at which immigrant women access motherhood is much lower, there is a rebound in maternal age at gestation parallel to that of national women.
The twin birth rate is maintained in a permanent and constant growth in Figure 6.
According to data from the National Institute of Statistics (INE) in Spain in the year 2013, 407,764 single births, 8,741 double births, 116 triple births and 2 births corresponding to quadruple gestation or greater were recorded for all ages. This represents a prevalence of 21.43 per thousand for twin pregnancies, 0.28 per thousand for triple pregnancies and 0.0049 per thousand for quadruple or greater pregnancies.
In Latin America, the frequency of twin pregnancy has remained stable in recent years. Brazil, in 1985, reported an incidence of 0.9%; Bolivia, in 1986, 0.8%; Chile, in 1986, 0.84%; Ecuador, in 1996, from 1.04%, Argentina, in 1997, from 0.99%, and in Venezuela, between 1976 to 1999, from 0.5 to 1.2%.17
In Mexico there are about 2.7 million births per year; of them, one in every 90 is twin pregnancies.18
In the INPer, which is a reference center for high-risk pregnancy, in the period from 1996 to 2000 the frequency of live neonates resulting from twin pregnancies ranged between 4.8 and 6.5%, which increased to around 10% during the period from 1996 to 2000 period from 2001 to 2010.19
In twin gestation, the earliest possible differentiation of chorionicity and zygosity is a priority. Zygosity will be responsible for genetic diseases and chorionicity will be responsible for the risk of complications during pregnancy and delivery in Figure 7.20
75% of twin pregnancies originate from two different oocytes, therefore they will always be bi-amniotic and bi-chorionic, although on a small number of occasions chorionicity can be confused because the placentas are practically fused with each other.
Monozygotic pregnancies represent 20-25% of twin pregnancies. The degree of fusion and the differentiation into one or two amniotic bags will depend on the moment in which the fertilized oocyte is divided. The longer the time of delay in the division, we will find a lesser degree of differentiation and a greater degree of fusion between twins Figure 8 and Figure 9.21
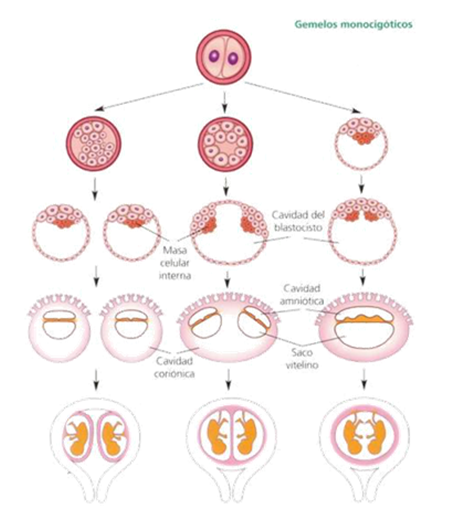
Figure 8 Monozygotic twin gestation division sadler TW Langman. Mediac embryology. 7th ed. Bs As. Ed. Pan American 1996.
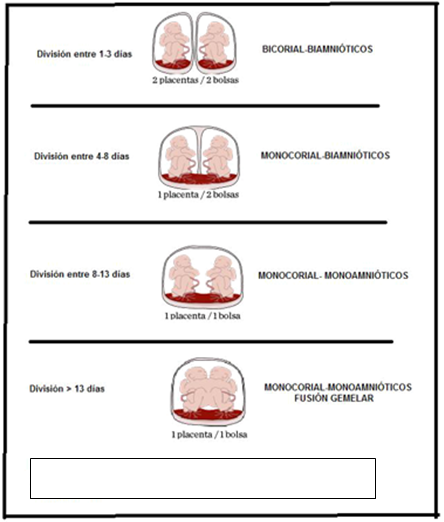
Figure 9 Placentation in monozygotic twin gestation occurs depending on the time the division occupies after fertilization.
Placentation in monozygotic twin gestation will occur depending on of the moment in which the division occurs after fertilization Figure 9.22,23
The longer the time between fertilization and division of the zygote, the more twins will be fused, even sharing organs. Which, depending on the type of union, can be:25,26
Globally, the calculated ratio of bizygotic and monozygotic pregnancies is 69% and 31% respectively,27 while the overall incidence of monozygotic twins is finds between 4 to 5 per 1,000 live births.28
The twin gestation presents a risk of maternal and fetal complications much higher than that of a single gestation and this will determine the antenatal and intrapartum management. Chorionicity is undoubtedly the main factor that determines the prognosis of pregnancy. And as we said before, zygosity is going to be responsible for genetic diseases and chorionicity will be responsible for the risk of complications during pregnancy and delivery.29
Maternal complications
Maternal complications are more frequent than in single gestation and include gestational complications and complications of childbirth and the puerperium. Maternal mortality is 2.5 times higher. Complications are conditioned by hormonal, hemodynamic (increased cardiac output and plasma volume) and mechanical factors. In addition, > 50% of the patients come from fertility treatments and advanced maternal age is more frequent. Gestational complications imply a greater need for hospitalization, immobilization in bed, fluid therapy, tocolytic treatments, and corticosteroids for fetal lung maturation. It is important to remember the added risk of acute lung edema and thromboembolic accident. Special attention should be paid in situations of fluid overload and prolonged rest. Obstetric hemorrhage is more frequent, and at the time of delivery it will be advisable to have a blood reserve due to the high risk of postpartum hemorrhage. The most frequent maternal complications are:
Fetal complications
Without a doubt, the greatest risk of multiple pregnancies is premature delivery; A 5.4 times higher risk of preterm birth has been described for twin pregnancies, and 9.4 times higher for triple pregnancies. In the United States and Canada, 10 to 14% of preterm births are attributable to twin pregnancies.31
Selective intrauterine growth restriction (IUGRs) is common in twin pregnancy; this is associated with a poor perinatal prognosis. Monochorionic twins have been associated with multiple neurological complications and sequelae.32
In Table 3 we can see the most frequent causes of neonatal morbidity in twins, which included all newborns, product of twin pregnancies, born at the National Institute of Perinatology, Isidro Espinosa de los Reyes (INPer, Mexico DF), during the period from January 1, 2007 to December 31, 2008. The data were obtained from the clinical file, with which the clinical history and evolution of the newborn were obtained until discharge. 654 newborns were included, the product of 327 pregnancies twins. In 2007 there were 152 twin pregnancies, which provided an incidence of 57.5 cases per 1,000 live births, while in 2008, with a total of 175 births, the incidence increased to 67.9 per 1,000 live births.33
Morbidity |
Frequency |
% |
IUGR |
361 |
55.2 |
Prematurity |
360 |
54.9 |
Pulmonary adaptation system |
218 |
33.3 |
Hyperbilirubinemia |
121 |
18.5 |
Transient tachypnea of the newborn |
75 |
11.5 |
Sepsis |
72 |
11 |
Congenital malformations |
56 |
8.6 |
Respiratory distress syndrome |
41 |
6.3 |
Necrotizing enterocolitis |
33 |
5 |
Apneas |
29 |
4.4 |
Gastroesophageal reflux |
26 |
4 |
Patent ductus arteriosus |
17 |
2.6 |
Suction disturbances |
12 |
1.8 |
Hypoglycemia |
9 |
1.4 |
Injuries associated with the birth route |
7 |
1.1 |
Bronchopulmonary Dysplasia |
6 |
0.9 |
Table 3 Neonatal morbidity of twins
Monochorionic pregnancies present an obstetric and perinatal risk greater than bicorial.34
Thus, monochorionicity implies an increased risk of stillbirth and fetal loss before week 24, selective intrauterine growth restriction (IUGRs), and neurodevelopmental disorders during childhood.35,36
To the complications typical of all multiple pregnancies, biamniotic monochorionic pregnancies add their specific complications (Table 4), such as fetal-fetal transfusion syndrome (FTFF), which appears in 10-15% of cases, the sequence anemia-polycythemia (SAP) in 5% of cases, IUGR in 10-15%, intrauterine fetal death of a single twin and the reverse arterial perfusion sequence or TRAP sequence.37,38
STFF |
10-15% |
IUGRs |
10-15% |
SAP |
5% |
MFI-TRAP |
1% |
Table 4 Specific complications of monochorionic pregnancies
The mechanism that currently seems to explain the development of many of the complications associated with monochorionic pregnancy seems to have its origin in the hemodynamic imbalance produced by the specific pattern of vascular anastomoses in monochorionic gestation, which interconnect the circulation of both fetuses, as well as to the unequal distribution of the placental territory between the two.38–41
Monochorionic twin pregnancy complications are grouped into 4 main types of clinical problems: chronic transfusion, acute transfusion, growth restriction, and discordant malformation. The interrelationships between these complications are illustrated in Figure 10.42
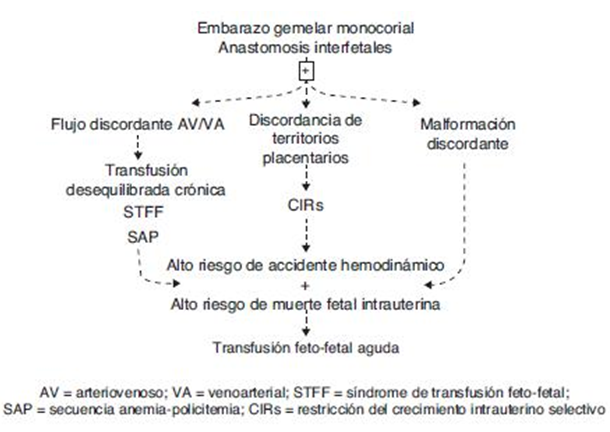
Figure 10 Complications of CM and their interrelationships.42
The fundamental characteristic of monochorionic twin pregnancies is the presence of placental vascular anatomosis, which can be arterio-arterial (AA), veno-venous (VV) or arterio-venous (AV). These placental connections cause fetal-fetal blood flow in both directions, representing a kind of third circulation between the twins, which is a unique feature in human pathology. Anastomoses can cause complications of monochorionic pregnancy by themselves or by combination with other factors, such as discordance of the placental territories and/or fetal malformations.43
Given the potential development of these complications, ultrasound monitoring of monochorionic pregnancies every 2 weeks from week 16 is recommended to diagnose and treat developing fetal complications early.44,45
Monitoring and proper management of monochorionic pregnancies can be achieved with a comprehensive vision that should be guided by basic principles Table 5. The complexity and, in some cases, the overlapping of complications that monochorionic twins present can blur clinical decisions of such so that these basic principles are forgotten:46
1. Early diagnosis (before GA week 15) and ruling out associated malformations. |
2. Follow-up every 2 weeks (PFE, CM-LA, MCA Doppler) by the fetal medicine specialist. |
3. If there are suspicions, weekly follow-up. |
4. If the polyhydramnios / oligoamnios sequence is present and the diagnostic criteria for FTFF are met: immediate treatment. |
5. If the PFE is <p10 in a fetus = IUGRs: UA Doppler. |
a) Normal: expectant management. |
b) Abnormal: discuss options with parents. |
6. If there are no complications elective delivery at 36-37 weeks of GA. |
MCA: middle cerebral artery; IUGRs: growth restriction selective intrauterine; CM-LA: maximum column of amniotic fluid; |
GA: gestational age; PFE: estimated fetal weight; FTFF: syndrome feto-fetal transfusion; UA: umbilical artery. |
Table 5 Basic principles of the management of monochorionic pregnancies
There is clear evidence that the diagnosis of twin pregnancy improves with the routine use of ultrasonography. The same occurs with the diagnosis of chorionicity, estimating that in the first or second trimester (<16 weeks) ultrasonography will determine chorionicity in 100% of cases.47
The ideal time to assess the chorionicity of a twin pregnancy is between 11 and 14 weeks.48
In early ultrasound before week 11, two yolk vesicles can be seen, although early diagnosis of amnionicity from the number of yolk vesicles is not always accurate Figure 11.
With the ultrasound between weeks 11 and 14 is the ideal time to evaluate the chorionicity of a twin pregnancy. In bichorial gestations the lambda sign (λ) is always present Figure 12.49
The biamniotic monochorial gestation (one placenta with two amnotic sacs) can be differentiated by the “T” sign that it presents at the junction of the two amniotic sacs in Figure 13. The “T” sign or fused amnion without chorion at the base of the sac loses sensitivity after 16 weeks. Other ways to diagnose bichorionicity are the presence of separate placentas and different fetal sexes; which combined, give a sensitivity and specificity greater than 90%.50
If it is not possible to define chorionicity, it is recommended to classify the pregnancy as monochorionic to ensure adequate control and avoid the non-investigation of complications associated with monochorionicity.
In monoamniotic monochorionic pregnancy, the amniotic cavity is unique and no membrane is observed between the fetuses in Figure 14.
Above week 14, the chorionicity study becomes uncertain and the lambda sign may disappear.
Gestation dating is done from the largest CRL to avoid underestimations in the case of initial restricted growth. The average growth difference in the first quarter is 3-5%.
Differences in CRL> 10% increase the risk of adverse perinatal outcome, both in monochorionic and bichorial pregnancies (fetal death, gestational loss, chromosomal or structural abnormalities, and weight difference), although the predictive value is low and the clinical utility is limited. Follow-up in CRL mismatches >10% at 11-13.6 weeks is as follows.
Follow-up in bichorial pregnancies with discordance CRL>10%
Routine aneuploidy screening (combined twin gestation/TN test+maternal age in triple gestation) and if the result is low risk for all fetuses (>1/250) perform a first trimester genetic sonogram of the smaller fetus to recalculate the risk. If low risk persists, an isolated CRL mismatch is not considered an indication for an invasive procedure. Although the need to perform an early echocardiography/early morphological echo (16 weeks) in the fetus of smaller size.51
Follow-up in discordant monochorial pregnancies CRL>10%
A routine aneuploidy screening is performed (combined twin gestation/TN test+maternal age in triple gestations. Single risk gestational). If low risk, an isolated CRL mismatch is not considered an indication for an invasive procedure. In monochorionic pregnancies with discordant CRL, the possibility of aneuploidy (concordant or heterokaryocytic) is less likely. Furthermore, the first trimester genetic sonogram is not applicable because the presence of secondary ultrasound markers (RT and especially a DVR-A) in some of the fetuses is a more frequent finding and in principle attributable to underlying hemodynamic disorders.
An early echocardiography should be performed (of both fetuses as in any monochorial pregnancy)/early morphological echo in the smallest fetus (16 weeks).51
Screening for aneuploidy
Aneuploidies are chromosomal alterations in which the number of chromosomes of that subject is not a multiple of the basic number of the same group of individuals. In this sense and from a theoretical point of view, we could find nullisomies (when the 2 homologous chromosomes are missing, 2n-2), monosomies (when a 2n-1 chromosome is missing), disomies (when the number of chromosomes is adequate, but 2 specific chromosomes they come from the same parent, which causes a disorder known as disomic uniparental inheritance), trisomies (2n + 1 chromosomes) and pentasomies. Tetrasomies appear in rare cases published in the bibliography where there are 2 or 3 extra chromosomes, always on the sex chromosomes.52
The most frequent aneuploidies in humans are monosomies (monosomies of autosomal chromosomes are not compatible with life), in particular Turner syndrome (45XO), and trisomies. For dysomic uniparental inheritance there is no screening test (they are detected by chance after performing chorionic biopsy and subsequent amniocentesis or by family history).52
There is no screening test during pregnancy for sexual trisomies (47XXY, 47XXX and 47XYY) and the other most common trisomies in humans, such as Edwards syndrome (trisomy 18) or Patau syndrome (trisomy 13), are incompatible with life and present multiple major malformations detectable by ultrasound. Therefore, Down syndrome is the most common disabling aneuploidy that we actually screen for during pregnancy. For this reason, in general terms, when we talk about aneuploidy screening we refer more commonly to Down syndrome screening, although with experience we know that Down syndrome screening also helps us to detect most of Turner's syndromes. Trisomies 18 and 13 and sometimes other aneuploidies.52
The screening of choice is the first trimester combined test:53 maternal biochemistry (PAPP-A and free ß-hCG applying a correction factor for each marker) between 7.6-13.6 weeks (preferably between 8-10 weeks) and ultrasound (TN) between 11.2-13.6 weeks (CRL between 45-80mm), preferably at 12 weeks, associated with maternal age. In the case of the pregnancy from oocyte donation, the maternal age to be considered will always be that of the donor. When the CRL of the older fetus measures between 80 and 84mm, combined screening is still feasible, but only if the maternal chemistry has been obtained up to 13.6 weeks (CRL up to 80mm).
Regarding the technique of NT measurement, it should be said that there are no differences in the distribution of NT between single or twin fetuses. TN screening is specific to each fetus, so incorporating NT measurement has been the most widely used method for aneuploidy screening.54,55
Bicorial gestations: The combined screening allows an estimation of the risk of trisomy 21 and trisomy 18/13 for each fetus based on its NT, always assuming that they are dizygotes.
Monochorionic pregnancies: since the risk of aneuploidy is the same for the 2 fetuses, as they are monozygotes, combined screening allows a single gestational risk estimate for trisomy 21 and trisomy 18/13 that is calculated using the mean NTs of the fetuses.
Combined test of the first trimester
The first-trimester combined test shows a trisomy 21 detection rate close to 90%, similar to the detection in single pregnancies, with a false positive rate of 5-6% of pregnancies.
A recent study has evaluated the calculation of the combined risk of the first trimester in twin pregnancies. Combined screening in twin gestations appears to be a good method for the detection of Down syndrome with a high detection rate and an acceptable false positive rate in Table 6.56
|
N |
> 35 years % |
Average EG in days to the extraction |
NT21 |
Median βhCG MoM in no affections |
Median PAPP-A MoM in not affections |
Median TN MoM on not affections |
TFP% per fetus |
TD % |
Goncé and cabbage |
161 |
15 (> 37) |
77 |
4 |
1.72 |
2.01 |
1.05 |
3.5 |
100 |
2008 |
|||||||||
Chasen and cabbage |
519 |
46.5 |
- |
7 |
0.97 |
1.12 |
- |
7 |
100 |
2007 |
|||||||||
Goncé and cabbage |
100 |
36 (> 34) |
77 |
3 |
1.57 |
1.96 |
1.02 |
3.6 |
100 |
2007 |
|||||||||
Spencer and cabbage |
206 |
- |
85 |
4 |
2.15 |
1.93 |
- |
6.9 |
75 |
2003 |
|||||||||
Orlandi and col * |
30 |
- |
84 |
7 |
1.72 |
1.61 |
0.9 |
10.6 |
- |
2002 |
|||||||||
Prats and cabbage |
447 |
30.6 |
67.2 BC/70.7 MC |
two |
1.74 BCC/1.44MC |
1.72 BC/1.51MC |
0.97BC/0.98 MC |
5.7 BC/4.4 MC |
100 |
2012 |
|
|
|
|
|
|
|
|
|
Table 6 Detection rate of the combined test in multiple gestations
*Pregnancy achieved through TRA
BC: Bicorial; MC: Monochorial; TFP: False Positive Rate; TD: Detection Rate; EG: AgeGestational; MoM: Multiples of the Median; N: Number of cases; TN: Nuchal Translucency; βhCG:
Free beta fraction of human chorionic gonadotropin; PAPP-A: Plasma Protein A associated with pregnancy; T 21: Trisomy 21.
It has been estimated that for a false positive rate of 5%, the detection of trisomy 21 with the combined screening is 90%; for a 2% false positive rate, the detection is approximately 80%.51,57,58
Multifetal gestation (3 or more fetuses) with CRL 45-84: maternal biochemistry is not applicable in this case because of the fetal number.
Ultrasound screening isolated with TN + maternal age (in case of oocyte donation, the maternal age to be considered as we said above will be that of the donor).
Twin pregnancies with CRL of the older fetus between 80-84 mm without the possibility of applying first trimester biochemistry (maternal analysis not performed before 14.0 weeks). Isolated ultrasound screening with TN + maternal age will be used (in the case of oocyte donation, the maternal age to be considered will be that of the donor).
In the last 20years, ultrasound has played a fundamental role in identifying the high-risk group for trisomy 21. Increased nuchal translucency (TN) between weeks 11 and 14 is the most effective ultrasound marker for the detection of trisomy 21 and other chromosomal alterations. During the last 15years, many works have focused on the methodology for the measurement of nuchal translucency and the development of algorithms necessary for the calculation of individual risk for trisomy 21, by combining NT with maternal age and other ultrasound markers.59
Isolated ultrasound screening with TN + maternal age has a lower trisomy 21 detection rate (75%) and a higher false positive rate (5% for each fetus in DC and 8% in MC/eg FP rate of 15% in a triple CT gestation).60
-Twin gestation starting control> 14.0 weeks (CRL of the largest fetus> 84 mm): the second trimester biochemical screening will be applied, preferably the quadruple test: fß-hCG) + AFP + uE3 + inhA associated with 36 maternal age (in the case of oocyte donation, the maternal age to be considered will be that of the donor). It will be carried out preferably at 15-18 weeks, but is applicable until 19.6 weeks. It allows the calculation of the risk of trisomy 21 and trisomy 18/13 of the entire pregnancy, applying a correction factor for each of the markers. This quadruple test has a lower sensitivity than in single pregnancies (65%) and a higher rate of false positives (10%). It is not applicable to pregnancies with more than 2 fetuses.51
There is little evidence on the usefulness of second trimester ultrasound markers in twin gestation (absent nasal bone, ductus venosus with absent or reverse flow in atrial contraction (DVR-A) and tricuspid regurgitation (TR) to modify the risk of trisomy 21 of the combined test, or to redefine the risk of ultrasound screening in pregnancies with more than 2 fetuses (TN), in bichorionic pregnancies it probably has the same utility as in single pregnancies.
In monochorionic pregnancies it is not indicated because hemodynamic markers are less applicable, since RT and especially DVR-A in some of the fetuses is a more frequent finding than in dichorionic or single pregnancies, and in principle it is attributable to hemodynamic disorders secondary to the existence of vascular anastomoses. Given the evidence of DVR in a monochorionic pregnancy in the first trimester, a greater risk of later appearance of TFF should be considered rather than an increased risk of aneuploidy.
Indications of the 1st trimester genetic sonogram for modify the risk of trisomy 21.
It is indicated in the smaller fetus in bichorial pregnancies with discordant CRL> 10% and an established low risk of aneuploidy.
There are other relative indications that can be applied selectively when the pregnant woman requests more information, such as: Dichorionic pregnancies with risks close to the limit (1/250) in trisomy 21 screening, in which it is preferred to reassess the risk before the invasive procedure is indicated
Invasive procedure indications
Types of invasive procedures
Chorionic biopsy: It will be the first option, except in individualized cases. It will be performed after a high-risk or discordant result in the first trimester screening in a dicorial gestation; it is especially important 38. Obtain the karyotype as soon as possible by chorionic biopsy. In the case of a discordant anomalous karyotype, early selective feticide significantly reduces the risk of the procedure.
Number of samples
In dicorial pregnancies: two samples must always be obtained. In the case of monochorionic pregnancies, as it is the result of a single zygote, obtaining a single sample is sufficient.
Amniocentesis
It will be carried out in gestations with gestational age ≥16 weeks. In very selected cases of diamniotic monochorionic pregnancies with early discordant ultrasound abnormality (for example discordant cystic hygroma with risk of monosomy X) compatible with heterokaryocytic gestation, amniocentesis will be performed to ensure obtaining two different samples.
Number of samples:
Risk of the invasive procedure
Several studies have shown that when performed by experienced operators, both amniocentesis and chorionic biopsy present a similar risk of pregnancy loss, and approximately 1% higher than the baseline risk of the pregnancy itself.51,61
The calculation method used by Fetaltest v3.1 has adopted the most recent contributions from other groups,62,63 and has the following characteristics:
Specificities of the a priori risk
The risk of the fetus being a carrier of a Trisomy depending on the maternal age in single gestations (Ru), is obtained from population studies.64 In these pregnancies, the risk that the fetus is a carrier of a trisomy coincides with the risk that the pregnancy is affected. However, in twin pregnancies, none, one or both fetuses may be affected, so the risk of pregnancy (Rg) does not have to coincide with the risk of each of the fetuses (Rf), which will depend on zygosity, so we can obtain:
In monozygotic twins, both twins will share the same chromosomal endowment, so the risk of each one and of the pregnancy would be the same and equal to that of single pregnancies.
Rmonocogotica = Rfeto1=Rfeto2=Ru
In dizygotic twins, the risk of chromosomal diseases of each fetus is independent of the risk of its co-twin, so it can be assumed that a fetus has the same probability of being affected as if it were single, and the other fetus would have the same probability of recurrence in single pregnancies (that is, that of a single fetus increased by 0.42%, at the end of the gestation). Hence, three options can (and should) be calculated:
As we do not know the zygosity of each twin pregnancy a priori, it will be necessary to perform the calculations of these four risks, which represent probabilities and, as was done in simple pregnancies, in order to be able to use them it will be necessary to transform them into "odds", which will be used in the final risk calculation.
Particularities of biochemical markers
The calculation of the probability ratio based on the biochemical markers is carried out in the same way as in single pregnancies, although ideally a set of population parameters obtained from twin pregnancies should be used. As such a set of parameters is not available for affected fetuses, it is necessary to use the population parameters of single gestations, which requires making corrections to the values obtained from these measurements.65
In twin pregnancies unaffected by trisomies, it could be expected (assuming that each twin contributes to the concentration of markers just as a single fetus would) that the mean of biochemical markers would be twice that of single pregnancies, that is, 2 MoM For this reason, a correction of the values of the biochemical markers has traditionally been carried out, consisting of dividing by 2 the serum concentration of these markers. However, many studies suggest that twin pregnancies do not actually usually have exactly twice the concentration of the markers as single pregnancies. In addition, a recent study has shown that the distribution of biochemical markers in twins with respect to single gestations varies, in addition to depending on chorionicity.
For PAPPA: log (k) = 0.1552 + 0.0059 xEG - 0.0001 xEG2
For B-HCG: log (k) = 0.2340 + 0.0079 xEG - 0.0002 xEG2
For PAPPA: log (k) = 0.2702 + 0.0048 xEG - 0.0001 xEG2
For B-HCG: log (k) = 0.2636 + 0.0029 xEG Where EG is gestational age expressed in days.
Fetaltest V3.1 uses these corrections for biochemical markers in twin gestations.
Although the incorporation of biochemical markers to the screening of first trimester chromosomal diseases in twin pregnancies is still far from the subtleties achieved in single pregnancies, there is some evidence that, even with the limitations outlined, the use of biochemical markers in twin gestations Twin pregnancies increase the efficiency of screening.
Particularities of ultrasound markers
Contrary to what happens with biochemical markers, ultrasound markers (and the CRL measurement) are specific to each fetus, which has some implications for screening:
A much more complex calculation method of the probability ratio related to NT, using a multivariate Gaussian model that takes into account the correlation between both measures. According to this model, three LRs would be calculated in all twin pregnancies:
To perform these calculations, the same general formula of the multivariate Gaussian model is used, based on the population parameters of single gestations, with the exception of the correlation coefficient between the TN measurements as Cuckle et al.66 have estimated at 0.45. Fetaltest V3.1 uses this calculation method, but uses the correlation coefficient observed in our own casuistry, which is 0.5371.
Particularities of the final risk calculation.66
In order to make an accurate calculation of the risk of twin pregnancies, it would be necessary to know the zygosity prenatally, and this is unknown. However, with the clinical and epidemiological data it is possible to carry out a estimation of the probability that a specific twin pregnancy is mono- or dizygotic, based on the ultrasound diagnosis of chorionicity, the sex of the fetuses, whether the pregnancy was spontaneous or through assisted reproduction, maternal age and ethnic origin. Thus, when a pregnancy is monochorial, it can be assumed that in 100% of cases it is monozygotic. In case of dichorionicity.
If both fetuses differ in sex, there is a 100% probability that the pregnancy is dizygotic.
For fetuses of the same sex
If pregnancy was achieved by in vitro fertilization and multiple embryos were transferred, there will be a 99.3% probability (obtained from epidemiological studies) that it is dizygotic, and 07% that it is monozygotic. If a single embryo was transferred in IVF, the probability of monozygosity will therefore be 100%.
If pregnancy was achieved spontaneously, the proportion of mono- and dizygosity depends on maternal age and ethnic characteristics, and can be obtained from calculations based on national pregnancy and delivery registries, such as those presented in the publication by Cuckle et al.66
With these data, the probability that the pregnancy is monozygotic (Pm) or dizygotic (Pd) will be obtained, in the form of a proportion.
To make the final calculation of the specific risk of each fetus, the 4 a priori risks obtained in section 1 (expressed in odds) are multiplied by the corresponding LRs obtained in section 3, and they average as a function of the probability that the pregnancy is mono- or dizygotic.
Thus, the probability that fetus1 is affected will be: Rfinalfeto1 = Pd * (Rdicigoticaf1 * LRf1 + Rdicigoticabos * LRambos) + Pm * Rmonozygotic * LRambos
And the probability that fetus 2 is affected will be: Rfinalfeto2 = Pd * (Rdicigoticaf2 * LRf2 + Rdicigoticabos * LRambos) + Pm * Rmonozygotic * LRambos
Therefore, this new calculation method allows obtaining the specific risk of each fetus in all twin pregnancies.
Once the risks of each fetus have been obtained as a function of NT, these are modified by the LRs dependent on the biochemical markers to obtain a final risk. Branches.67
In 2006 in the US it was estimated that the twin birth rate corresponding to ART represented 1% of all births and 18% of all twins. Of the births achieved by ART, 48% were twins.68
The possible association between congenital defects of all kinds, malformations and chromosomes, and ART continues to be a subject of wide controversy in the literature, despite the progressive increase in children born with these procedures in the last 25years. There are studies that deny the evidence of an increase and others do prove the presence of various defects. Underlying the issue in all of them is a lack of data uniformity, small samples, and a lack of adequate control groups. The high efficacy and safety offered by combined screening is beyond doubt, both in the extensive recent literature and in our own cases, and especially in the group of pregnant women after assisted reproduction treatments, with a very acceptable detection rate. As expected, A slight increase in false positives is assumed with respect to spontaneous pregnancies, due as the majority of authors agree, to the decrease in the PAPP-A figure that is associated with pregnancies after ART.69
The article by Amor et al also reaches this conclusion, with the broadest casuistry published to date.70
On the other hand, pregnancies conceived by IVF present a high percentage of dichorionic twins71–73 causing a double hormonal production of PAPP-A and fßhCG to be generated, so that any dysfunction of increase or decrease of these hormones, as has also been described in simple pregnant women through IVF, is doubly increased or decreased. This fact makes the ultrasound study of twin pregnancies necessary to determine their chorionicity (echographic sign lambda (λ) bichorionic or T monochorionic) already described by Sepulveda,74 and the calculation with differentiated normality curves depending on whether it is mono- or bichorionic twins.
In the case of twin pregnancies achieved through ART, the development of normality curves for this subgroup of IVF twins is required or, failing that, the application of possible differentiated correction factors in the calculation of prenatal risk.75,76
Given the evidence of these differences, in pregnancies conceived by IVF, various working groups have applied correction factors to the values of PAPP-A and fßhCG to reduce this effect.77–79
There is no unanimous application of these factors given the variety of diagnostic determination methodologies and calculation programs. For this reason, it is recommended that each group carry out preliminary studies of their results to obtain the necessary correction factor in each case.80
It is therefore a priority that in order to calculate the risk of first-trimester prenatal screening in twin pregnancies, it is necessary, on the one hand, to establish the chorionicity of the twins (mono- or bichorionic) and, on the other hand, to draw up normality curves. Differentiated for twin pregnancies conceived by IVF. Since in the curves carried out by Ramírez C et al, it has been observed that the difference between the values of the concentrations of PAPP-A and fßhCG of spontaneous pregnancies and IVF is not constant, and depends on the gestational age at which the test is performed analysis. Thus, until a significant number of 1000 pregnant women are available to perform these differentiated curves, as indicated by the FMF.81
The study by82 shows a similar increase in the number of false positives in the ART group, being 6.84% compared to 3.79% of the spontaneous ones (in the aforementioned article, it represents 10.1 compared to a 4.0%). Likewise, lower PAPP-A figures are obtained, although without statistical significance, as in other publications.83–86
There has been a slight increase in invasive procedures in pregnant women after ART, but this number is always a much lower number, even if the invasive diagnostic test was only indicated for maternal age over 35years, a situation that is relatively frequent in women who choose to TRA. Therefore, by means of combined screening, the global number of invasive procedures, of vital importance in these, is reduced.
Pregnancies in which the slightest added risk is a special concern for the pregnant woman and her partner. In the literature, there is a wide use of combined screening in pregnancies achieved after ART.87,88
It would appear that performing combined screening in twin pregnancies does not improve the sensitivity of ultrasound screening, but it does reduce the number of false positives.89
Free fetal dna in maternal blood. Non-invasive test
General limitations
First of all, we must be clear that this test is a screening, it is not a diagnosis. In the event of a positive result, an invasive diagnostic test should therefore be considered.
Mosaicism
Generalized mosaicism: it is defined as the presence of two or more karyotypically different cell lines, both in the placenta and in the fetus. In cases of generalized mosaicism, there is a possibility that the non-invasive test result will be a false positive or a false negative, depending on the origin of the cell-free DNA.
Mosaicism confined to the placenta: is the presence of two or more karyotypically different cell lines that are confined to the placenta and are not present in the fetus. In cases of mosaicism confined to the placenta, there is a possibility that the test result will be a false positive.
Fetal mosaicism: defined as the presence of two or more karyotypically different cell lines that are present in the fetus, but not in the placenta. In cases of fetal mosaicism, there is a possibility that the test result will be a false negative.
This test cannot replace ultrasound scans performed in the first and second trimesters, which are essential during pregnancy. The non-invasive test is not capable of detecting abnormalities of the fetal organs such as those of the heart or the brain, so ultrasound monitoring of pregnancy is essential.
Advantages of the Test
Non-invasive test for singleton pregnancies
It allows the detection of trisomies 13, 18 and 21, and aneuploidies of the sexual pair (monosomy X, XXX, XXY, XYY), in addition to fetal sex. With some platforms it is possible to expand the study with a panel of 3 or 5 microdeletions associated with intellectual deficit: Di George (del22q11.2), Prader-Willi/Angelman (del15q11.2), Cri du Chat (5p-), Wolf- Hirschhorn (4p-) and 1p36 deletion syndrome.
In Table 7 we can see a comparative analysis between the main platforms that are currently available.
Company to |
Test |
Minimum week gestation |
Technique |
Database (patients) |
Sensitivity |
Sensitivity |
Sensitivity |
Sex |
Term minimum (days workings) |
ad T21 |
ad T18 |
ad T13 |
|||||||
BGI |
NIFTY |
10 |
MPS (Sequencing massive ion parallel) |
211883 (Nov. 2013) |
99.65% |
99.66% |
100% |
Yes |
10 |
Natera |
Panorama |
9 |
SNPs |
1,194 |
> 99% |
> 99% |
> 99% |
Yes |
10 |
Verinata |
Check |
10 |
MPS (Sequencing n massive parallel) |
2010 |
> 99.9% |
97.40% |
87.50% |
Yes |
5 |
Sequenom |
MaterniT21 |
10 |
MPS (Sequencing n massive parallel) |
5,698 |
99.10% |
> 99.9% |
91.70% |
Yes |
5-7 |
Ariosa |
Harmony |
10 |
MPS (Sequencing n massive parallel) |
> 6,000 |
> 99% |
> 98% |
80% |
Yes |
5-7 |
LifeCode xx (partner of Sequeno m) |
Prena Test |
9 |
MPS (Sequencing n massive parallel) |
- |
99.80% |
- |
- |
- |
10 |
Table 7 Comparison of specificity and sensitivity data for different platforms
Non-invasive test for twin pregnancies
It is possible to carry out the non-invasive test in twin pregnancies, as long as there is a sufficient amount of DNA from each of the fetuses in the maternal blood plasma. Although in single pregnancies the measurement of the fetal fraction is a necessary quality control to carry out the test, in twin pregnancies this quality control becomes even more important, requiring a minimum value of 8% of fetal DNA to be able to detect fetal trisomies (a value that corresponds to twice the minimum required in singleton pregnancies, 4%).90
What is detected?
Detection is focused on Trisomies 13, 18 and 21, as well as the presence/absence of the Y chromosome.
Limitations
It is not possible to detect aneuploidies in the sexual pair, as well as fetal sex.
It is only possible to see the absence or presence of the Y chromosome, but it is not able to determine if it corresponds to one or both fetuses.
In the case of twin pregnancies, the available levels of DNA from each fetus in maternal blood are lower than those present in a single pregnancy.
Verinata has validated the study by analyzing 115 maternal blood samples from twin pregnancies and as a result Illumina (formerly Verinata Health) publication “Accurate Aneuploidy Detection in Twin Pregnancies using the SAFER Algorithm”, Data on File. got:
In twin pregnancies, it must be taken into account whether it is monochorial or bicorial, since the non-invasive test presents important differences between them:
Monochorionic twins share a placenta, they are identical, so the calculated risk is unique and the same for both. Nuchal translucency values can be altered by Fetal Transfusion syndrome.
In bichorial twins, the risk of each of them is calculated. It should be known that the risk of chromosomal alterations in a twin pregnancy is the accumulation of the risk in two single pregnancies, and therefore, greater.
In monochorionic twin pregnancies, the non-invasive test makes it possible to detect whether the fetuses have any of the aforementioned alterations with great accuracy. In the case of bichorial twin pregnancies, a negative result applies to both fetuses, while a positive result indicates 56 that one of the fetuses may present some alteration but it is not possible to know the state of the other fetus without resorting to invasive techniques. Table 8.91
Fetal karyotype |
Risk score from cfDNA testing |
|||
n |
trisomy 21 |
trisomy 18 |
trisomy 13 |
|
Monochorionic euploid (in m 8-1) |
84 |
<1:10,000 |
<1:10,000 |
<1:10,000 |
Dichorionic euploid (n = 109) |
97 |
<1:10,000 |
<1:10,000 |
<1:10,000 |
Monochorionic trisorny 11 (n :z. L) |
1 |
>99% |
<1:10,000 |
<1:10,000 |
Dichorionic concordant trisorny 21 (n=1 ) |
1 |
>99% |
<1:10,000 |
<1:10,000 |
Dichorionic discordant trisomy 21 (n = 8) |
6 |
>99% |
<1:10,000 |
<1:10,000 |
1 |
72% |
<1:10,000 |
<1:10,000 |
|
1 |
0.5375 |
<1:10,000 |
<1:10,000 |
|
Dichorionic discordant trisomy 18 (n = 1) |
0 |
|||
Dichorionic discordant trisomy 13 (n = 3) |
1 |
<1:10,000 |
<1:10,000 |
>99% |
Table 8 Risk scores for trisomies by cfDNA testing of metarnal plasma in mano- and dichorionic twin prcgnancies91
Regarding trisomies 13, 18 and 21, the possible results are reported as follows:
Regarding the presence of the Y chromosome, the information provided is simply: "detected" or "not detected".
None.
None.
The authors declare that they have no conflict of interest.

©2021 Sánchez-Seiz. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.