eISSN: 2377-4304


Research Article Volume 10 Issue 3
1IVF, Kulakovs Scientific Centre of Obstetrics, Gynecology and perinatology, Russia
2Oxylipin lab, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Russia
Correspondence: Veronika Smolnikova, IVF, Kulakovs Scientific Centre of Obstetrics, Gynecology and perinatology, Moscow, Russia, Tel 7 (903)723 08 47
Received: June 07, 2019 | Published: June 28, 2019
Citation: Smolnikova V, Zorina I, Luiza I, et al. Role of genetic and molecular predictors in optimization of in vitro fertilization programs for selective embryo transfer. Obstet Gynecol Int J. 2019;10(3):230-234. DOI: 10.15406/ogij.2019.10.00448
Introduction: This research presents data about embryo capability for implantation based on the study of changes in metabolomic profile of embryo culture media, consumption of constituents of embryo culture media and anamnestic factors predisposing to aneuploid embryos. Determination of glucose and glutamate concentrations in spent media samples from embryos collected on day 5 is a new noninvasive method of assessment embryo quality to efficiency improvement of IVF outcomes.
Material and methods: 96 couples underwent IVF program with preimplantation genetic testing (PGT). Spent media samples from embryos that resulted in pregnancy and delivery and samples from embryos that failed to implant were individually collected on day 5, and evaluated using mass-spectrometry.
Results: Сorrelation was identified between metabolomic profile, implantation potential of embryo and ploidy of embryos.
Conclusions: Analysis of composition of embryo culture media maid by method of metabolomic profiling can result in more specific selection of embryos for further transfer.
Problems with conception are still relevant for couples of reproductive age throughout the world. For some of them, the only chance to have a child is using assisted reproductive technologies (ART).
There are many factors which effect success of infertility treatment: ovarian reserve, patient age, infertility factor, concomitant gynecological and somatic diseases, timely detection of intrauterine pathology, endometrial morphology, determination of the implantation window.
Implementation of special methods of conception, constant improvement of drugs, personalized guideline-based treatment recommendations, modification of embryo culture methodology can lead to higher rate of successful treatment of infertility. However, this does not solve the problem of unsuccessful outcomes of IVF programs. Pregnancy rate does not exceed 50% even if woman has euploid embryo and normal characteristics of the endometrium before the embryo transfer (the endometrium thickness exceeds 8 mm).1,2
Assessing the morphological quality of embryos is a common method of selecting an embryo suitable for transfer. Embryologists use the blastocyst scoring system developed by Gardner et al.
This grading system has three separate quality scores for each blastocyst. The final score assigned for each blastocyst is composed of these three scores. Therefore the first number is the expansion score, a number from 1-6 based on degree of expansion and the hatching status. The ICM score is listed second as, A. many cells forming a cohesive epithelium, B. few cells forming a loose epithelium and C. very few large cells. The final score is the TE score (A. tightly packed, many cells, B. loosely grouped, several cells and C. very few cells).3–5
On the one hand there is always a risk that good quality embryos would not implant because they have aneuploid chromosome number.6 On the other hand poor quality embryos can lead to pregnancy and increase the number of live births.6
The world's largest studies reported that 10% cases of termination of pregnancy related to chromosomal abnormalities: chromosomes either missing from or extra to the normal pair.7–12 For the last 10 years Preimplantation genetic testing (PGT) has been reliable method of diagnosticating genetic abnormalities of embryos before transferring them to the uterine cavity.13 The rates of successful implantation, live birth have increased recently while the number of miscarriages of patients with PGD has declined in comparison to patients with standard IVF.14–16 Despite the fact that such method of embryo selection improves morphological quality there are certain disadvantages that should be mentioned. Such as chance of negative influence to embryo during trophectoderm biopsy, of mosaicism during interpretation of results, chance of self correction and selective apoptosis of embryo on early stages of development and also high cost of research and inability to provide research in lack of material.17 Besides PGD cannot completely exclude a chance of existence of genetic numerical aberration and point mutation, that could affect implication of embryo and lead to development of congenital malformation.18–23
That is why improvement of modern approaches to investigation of biological processes that impair implantation24–26 and non-invasive diagnostic techniques of viability evaluation of embryos are of great importance.27–29
Omics technologies are new spheres of science allowing to investigate functioning of cellular structures from DNA and genes to Metabolites. Application of omics technologies helps to detect new molecular infertility biomarkers that lead to widening of diagnostic capability and improving IVF results.
Embryo culture medium containing information about metabolic activity, energy metabolism and condition of cell signaling pathway is unique subject of study.30 In view of the aforesaid it is relevant and promising to find out the predictors of successful implantations leading to pregnancy and live birth.
The Goal of research is optimization of ART methods by determining ability of embryo to successful implantation during selective embryo transfer to uterine cavity. On the bases of investigation of metabolomic profiles, namely glucose and glutamate consumption by embryo culture medium.
Retrospective and prospective study of 96 married couples who applied for an IVF-program with PGT. All patients were previously examined. Morphological assessment was performed on day 5 and then trophectoderm biopsy of blastocyst for PGD by using comparative genomic hybridization method (aCGH). The time needed to obtain the results of the genetic tests may not allow a fresh embryo transfer to be performed. The blastocysts cryopreserved immediately after the biopsy. To determine molecular genetic quality predictors of developing embryos, all samples of culture media were conveniently classified into groups depending on their genetic and morphological quality and outcomes of embryo transfer to the uterine cavity. Metabolic profiles of spent culture media were analyzed using mass-spectrometry
Entry criteria
Non-inclusion criteria
Exclusion criteria
Ovarian stimulation begins on day 2-5 of the menstrual cycle according to protocol with gonadotropins and gonadotropin‐releasing hormone antagonist (GnRH antagonists) with further PGD by using comparative genomic hybridization method (aCGH)
Program was conducting according to standard protocols with gonadotropins and gonadotropin‐releasing hormone antagonist depending to age of patient, ovarian reserve and hormonal state.
After 35h of HCG injection (dosage 10 000IU on follicular size-18mm or greater in maximal diameter.) oocytes were collected by transvaginal ultrasoundguided needle aspiration of the follicles under deep conscious sedation. For fertilization was used method ICSI to avoid effect of side DNA. On 5 day of cultivation after morphological analysis (scoring system developed by Gardner et al) embryos’ trophectoderm was biopsied and then cryopreservation of embryos is performed.
Metabolic profiles of spent culture media were investigated by mass-spectrometry to identify potential biomarkers of successful implantation.
In order to reveal factors effecting morphological quality of embryos in IVF programs method of logistic regression was used. Dichotomous variable was morphological quality of embryo either good or fair. According to logistic regression coefficients should not be correlated. In case of correlation coefficient is excluded.
Multifactorial allowed to determine influence of independent factors on dependent variable. As independent further factors were determined: age, menarche, regular menstrual cycle, BMI, anamnestic data, factor of infertility, quantity of IVF programs (ICSI), dosage of drugs and duration of stimulation, method of fertilization, quality of embryos and karyotype.
Step by step inclusion and exclusion of above mentioned variables allowed determining exact predictors effecting the result.
There were 306 PGT 56,9% (174) of them were of good morphological quaility, 37,9 % (116) were of fair morphological quaility and 5,2 (16) were of poor morphological quaility. In groups of embryos with good quality 40% (69) were with chromosomal abnormalities. In groups of embryos with fair quality 62% (72) were with chromosomal abnormalities and in groups of embryos with poor quality 23% (69) were with chromosomal abnormalities. In other words there were less aneuploid embryos after PGT in group of good morphological quality than in group fair morphological quality.
Within the patient group of under 34 years 172 embryo were examined for genetic abnormalities, 61% had euploid karyotype. 36 embryos were taken from patients of 35-39 years and 47 % of them have euploid karyotype (17). 98 embryos were taken from patients over 40 years old and 40% f them have euploid karyotype (40). It is obvious that there is direct correlation between age and ploidy of embryos.
Besides the correlation between wrong genetic embryo karyotype and external genital endometriosis and also type of infertility was revealed.
Taking into account change of composition of materials during research all profiles of spent culture media metabolites were divided in two groups - intraspecific and cross-species.
On 5 day of cultivation all embryos were divided in 4 classes according to morphological research and given morphological mark. Principal components analysis (PCA) was used to reduce size of multicomponent selections , data and for visualiztion of clusterization examples according to metabolites profile. Differences in profiles spent culture medium metabolites were revealed.
It is remarkable that all above mentioned classes were different to group of controlled culture medium (without cultivation of embryos). While embryos that stoped growing at early stages had no differences to controlled group.
Intraspecific comparison of embryos of one morphological class did not show any differences between metabolomic profiles. Just as well profiles of culture media of euploid and aneuploid embryos. While metabolomic profiles of different morphological classes have variations.
At the next stage, the analysis of the correlations between levels of metabolites in the spent culture media of patients after IVF(ICSI) program with PGD included in the study.
PCA allowed detecting significant differences between groups with different outcomes after selective embryo transfer. Сompounds were identified by chromographic retention time, molecular mass and fragmentation of the corresponding molecular ion.
With a qualitative comparative analysis of the parameters of the obtained mass peaks in spent culture media of implanted embryos concentration of L-Valine (118,0864 Da), L-Proline (138,0526 Da), alanyn-glutamine (218,1144 Da), Phenylpyruvic acid ( 165,0545 Da) и b-L-Fucosa-1(166,0866 Da) has decreased, and concentration of l-phenylalanine (166,0866 Da) has increased by comparison to spent culture media of unimplanted embryos. This result suggests that changes in embryo metabolism at the cultivation stage have significant importance for embryo implantation (Table 1).
Molecular mass, Dа |
Presumed molecule |
Concentration changes |
118.0864 |
L – valin |
18 times less |
138.0526 |
L – proline |
4100 times less |
218.1144 |
alanyn-glutamine |
19 times less |
166.0866 |
L – phenylalanine |
287 times more |
165.0545 |
Phenylpyruvic acid |
697 times less |
267.0257 |
b-L-Fucosa-1 |
3069 times less |
Table 1 Changes in the concentration of the investigated substances in the culture media of implanted embryos compared with non-implanted
Moreover during this research it was found out that glucose consumption level by first class embryos 1,75 times more than that of second class embryos and two times more than of third class embryos. There were no differences connected to this parameter between second and third class of embryos.
There is no reliable data about differences in glutamate consumption between embryos of all classes.
PGT showed that at average 50 % of investigated in research embryos have aneupoloud karyotype.
Intraspecific comparison revealed no differences between euploid and aneuploid embryos in consumption of glucose and glutamate (Figure 1) (Figure 2).
Average rate of glucose consumption by spent culture media for implanted embryos was 2,5 (2,0-3,4) nmol and for unimplanted embryos - 0,75 (0-1,6) nmol. Implanted euploid embryos consumed 3,4 times more glucose than that consumed unimplanted euploid embryos (Figure 3).
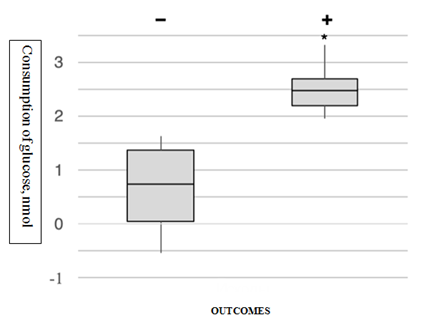
Figure 3 Glucose consumption by implanted (+) and non-implanted (-) euploid embryos 5 days of development. (The line in the middle of the “Box” is the median (50th percentile), the border is the first and third quartiles (the 25th and 75th percentile). The ends of the "whiskers" correspond to the minimum and maximum values. Extreme points (outliers) are data that go beyond the whiskers. * - p<0.05 (Mann-Whitney test).
Сonsequently high level of glucose consumption out of culture media can be predictor of successful implantation of embryo.
Metabolomics is an emerging “omics” science that has evolved from proteomics, genomics and transcriptomics. It systematically analyzes the inventory of metabolites.
Metabolomics are promising new approaches to assess embryo viability using non-invasive methods. A direct, simple and rapid analysis of spent culture media from blastocysts at the point of embryo transfer can quickly identify embryos with the best chance of achieving ongoing pregnancy. Methods like this, which take less than 20 min to perform, could dramatically improve the approach to embryo selection and live births.
Ray K. Iles et al. investigated 401 samples of spent culture media collected from embryo cultures at the time of embryo transfer, of which 136 were used to construct the predictive model. With a simple algorithm, it was possible to identify samples with the best chance of becoming an ongoing pregnancy.31
Ercan Baştu examined spent culture media using Raman spectroscopy from 31 women. Clinical pregnancy was predicted using Raman spectroscopy in 93% (14/15) of clinically pregnant patients, and in 62.5% (10 out of 16) of clinically non-pregnant patients. Adding Raman spectroscopic analysis of spent embryo culture media revealed that this approach may predict clinical pregnancy as an adjunct to morphologic evaluation.32
Pyruvate is the favorite substrate for cleavage stage mammalian embryos include the human and is able to support the development of human blastocysts,33 pyruvate and glucose uptake has previously been correlated with embryo viability.34
Based on that we can make a conclusion that our research is up-to-date ad necessary for development of assisted reproductive technologies.
None.
Author has no conflict of interest to declare.

©2019 Smolnikova, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.