eISSN: 2377-4304


Research Article Volume 1 Issue 3
1Department of Gynecological Oncology, Nanjing Medical University, China
2Department of General Surgery, Nanjing University, China
Correspondence: Jin-Hua Wang, Department of Gynecological Oncology Surgery, Jiangsu Cancer Hospital & Institute, 42 Baiziting Road, Nanjing, Jiangsu 210009, P.R. China, Tel 86-25-83283321, Fax 86-25-83283320
Received: November 06, 2014 | Published: December 10, 2014
Citation: Wu Y, Wang S, Wang Y, et al. Reversal effect of docosahexaenoic acid on taxol resistance in human ovarian carcinoma A2780/T Cells. Obstet Gynecol Int J. 2014;1(3):70-76. DOI: 10.15406/ogij.2014.01.00018
Docosahexaenoic Acid (DHA), a member of n-3 PUFAs, has been reported to have multiple beneficial anticancer actions. However, not much was known about the role in ovarian cancer. The aim of this study was to assess the effects of DHA on reversing drug resistance and explore the possible mechanisms in ovarian cancer. The results showed that DHA dramatically enhanced the sensitivity of cells to Taxol in A2780/T cells rather than A2780. Additionally, Taxol and DHA in combination could alter the cell cycle distribution and arrest the cell cycle in G0/G1 phase. We also found that DHA promoted the chemotherapeutic drug accumulation in cells and inhibited the expression of P-gp and other MDR related proteins, including BRCP, MRP and LRP. The activation of NF-κB and phosphorylation of P38MAPK were found markedly inhibited by pretreated DHA. To summarize, DHA could reverse Taxol resistance by inhibiting expression of P-gp and other MDR related proteins, as well as blocking NF-KB and p38MAPK pathways. DHA, a natural agent, might be widely used as a supplemented method in fighting against cancer in future.
Keywords: docosahexaenoic acid, ovarian carcinoma, taxol resistance, P-glycoprotein, nuclear factor-Κ-gene binding, P38 mitogen-activated protein kinase
n-3 PUFAs, n-3 poly unsaturated fatty acids; DHA, docosahexaenoic acid; EPA, eicosa pentaenoic acid; MDR, multi drug resistance; P-gp, P-glycoprotein; NF-κB, nuclear factor-κ gene binding; P38 MAPK, P38 mitogen-activated protein kinase
Ovarian cancer is the second most common gynecologic oncology in the world and ranks first in mortality with 14,270 deaths estimated to have occurred in 2014 in USA.1 Resulting from difficulties in early detection, 70% of cases are detected in stages III and IV. At the advanced stage, combination regimens containing taxanes (e.g. Taxol) occupy an important position in ovarian cancer following optimal tumor debulking surgery.2 Although most patients initially respond well to chemotherapy, 85% of them suffer the relapse, largely due to drug or multidrug resistance (MDR) and wide spread metastasis.2 Thus, owing to MDR and invasion, the mortality of ovarian cancer has not been significantly decreased with the emerging chemotherapy. The five-year survival rate is only 30% to 40%.3,4
N-3 polyunsaturated fatty acids (n-3 PUFAs), including docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), are essential fatty acids necessary for human health. Several epidemiologic and clinical studies have shown that DHA and EPA have beneficial effects on malignancies,5 such as breast cancer, prostatic carcinoma,6 and colon cancer.7
Besides inhibiting process of premalignant and malignant lesions and preventing weight loss in treatment,8,9 DHA has also been associated with improving sensitivity of tumor cells to various chemotherapeutic drugs without affecting cell viability,10 which means DHA could act synergistically with anticancer drugs. Although various beneficial effects of DHA were reported, to our knowledge, little attention has been paid to the role of DHA on ovarian cancer cells.
In the study, we investigated whether DHA could enhance the susceptibility of human ovarian cancer cells to Taxol. Besides, its reversal effect was through mediating over-expression of P-glycoprotein (P-gp). Moreover, we demonstrated that the mechanism of reversal effect also involved down-regulating the expression multidrug resistance-associated proteins and inhibiting the activity of Nuclear factor-κ-gene binding (NF-κB) and p38 mitogen-activated protein kinase (p38 MAPK) signaling pathway. This might provide a scientific basis for improving survival in patients with ovarian cancer.
Chemicals and reagents
Taxol was purchased from Taiji Pharmaceutical Co. Ltd (Sichuan, China). Rhodamine123 and DHA were obtained from Sigma Chemical Co. (St Louis, MO, USA). Paclitaxel Liposome was from lvyesike Pharmaceutical Co. Ltd (Jiangsu, China). Anti-P Glycoprotein antibody (ab3366) was from Abcam Biotechnology. Antibodies against β-actin, GADPH, multidrug resistance-related protein1/2 (MRP1/2), breast cancer resistance protein(BCRP), lung resistance-related protein (LRP) and horseradish peroxidase-linked (HRP) secondary antibody were from Nanjing Enogene Biotech Co. Ltd (Jiangsu, China), Antibodies against p38 MAPK, Phospho-p38 MAPK, NF-κB were from Cell Signal technology (USA). Cell apoptosis kit and cell cycle assay kit were from BIO-BOX Biotech Co. Ltd (Jiangsu, China). RIPA lysis buffer, icinchoninic acid assay (BCA) kit and Trizol Reagent RNA extraction kit were from Beyotime Institute of Biotechnology (Jiangsu, China).
Cell culture
The human ovarian cancer cell line A2780 was obtained from Enogene Biotech Co. Ltd (Jiangsu, China). The A2780/Taxol (A2780/T) cell line was induced by stepwise sequential exposure to increased concentration of Taxol. To maintain the drug-resistant phenotype, the A2780/T resistant cell line was finally incubated in Taxol-containing medium (800ng/ml). A2780 and A2780/T were cultured in RPMI1640 medium supplemented with 10% fetal bovine serum (FBS), 100U/ml penicillin and 100U/ml streptomycin (GIBCOBRL, Paisley, UK) at 37˚C in 5% CO2. Experiments were performed only after all the cells had been maintained in drug-free medium for two months.
Measurement of Cell Viability
Briefly, 1×105 cells in logarithmic phase were seeded in 96-well plates. After 24h preincubation, the medium was removed and replaced by various concentrations of DHA. Ten different concentrations for DHA were analyzed and each concentration was replicated in 3 wells. The medium was removed after 72h, and 15μl of 5mg/ml MTT solution was added to each well for 4h at 37˚C. After dissolving the resulting formazan product with dimethylsulfoxide (Sigma), an enzyme-linked immunosorbent assay reader was used to detect optical density (OD) values at 490nm for each well. The maximum safe dosage for DHA (survival rates of cancer cells were more than 90%) was used as optimal dose of DHA in A2780 or A2780/T.
Then, we performed MTT to investigate the cytotoxcity of Taxol in A2780 and A2780/T and each kind of cell was divided into two groups. Cells pretreated with optimal dose of DHA for 48 h were used as experimental group. Untreated cells were used as control group. According to same method, ten different concentrations for Taxol were analyzed and each concentration was replicated in 3 wells. The IC50 is defined as the dosage of drugs in which 50% of cellular death (50% reduction of absorbance at 490nm) after 72h treatment. Resistant index (RI)=IC50a/IC50b (a=A2780/T, b=A2780/T +DHA.)
Rhodamine123 accumulation assay
5×105 cells were seeded in 6-well plates. A2780/T cells were pretreated with 3µM Taxol (IC50 of A2780/T to Taxol) and various concentration of DHA (0-296µM) for 48h. Then, 5umol/L Rhodamine123 was added and the cells were incubated in the dark at 37˚C in 5% CO2. After 2 hours, cells were washed three times with ice-cold phosphate-buffered saline (PBS) and resuspended in PBS with 10% FBS. The fluorescence of intracellular Rhodamine123 was determined both by FACS scan FCM (Becton Dickinson, San Jose, CA) and fluorescence microscope (OLYMPUS, Tokyo, Japan).
Cell cycle analysis
Treated cells were centrifuged and suspended in cold PBS, fixed with 70% cold ethanol at 4˚C overnight. Cells were washed and resuspended in 1ml PBS for 15min to remove ethanol, then incubated with 100ul RNase A at 37˚C for 30min followed by staining with 400µl propidine iodide (PI) for 30min in the dark. Samples were analyzed for DNA content by FCM. In the study, 10,000 cells were counted for each determination.
Quantitative RT-PCR analysis
Pretreatment of drug (Taxol or DHA) for 48h, total RNA was extracted by RNA iso Reagent (Takara, Japan). Before reverse transcription (RT), samples were treated with DNase I. PCR primers for MDR1 (forward primer of 5′-CCCAGGAGCCCATCCTGT-3′, reverse primer of 5′-CCCGGCTGTTGTCTCCATA-3′) GADPH (forward primer of 5′-AATCCCATCACCATCTTCCA-3′, reverse primer of 5′-TGGACTCCACGACGTACTCA-3′). According to manufacturer’s procedure for relative quantification, quantitative RT-PCR was performed with power SYBR Green PCR Master Mix (Applied Biosystems, USA) using a Step One Plus™ Real-Time PCR System (Applied Biosystems, USA). The standard temperature profile was as follows: 95°C for 10 min followed by 40 cycles at 95˚C for 15s, 60˚C for 60s, 72˚C for 10s. The results were obtained from three reactions in each sample. The relative amount of each target was expressed as 2-ΔΔCT values.
Western blot
After washing with PBS, the total cellular samples were lysed in RIPA lysis buffer (Beyotime, Haimen, China) supplemented with 10mM PMSF on ice. Supernatants were collected after centrifugation at 12000rpm for 15 min at 4˚C. Protein concentrations were determined using the BCA protein assay kit. 50µg of total protein were electrophoresed in 10% SDSPAGE gels and transferred onto nitrocellulose membranes (Millipore, Bedford, MA) which were incubated with antibodies at 4˚C overnight. After washed by TBST, membranes were incubated with HRP-secondary antibody for 1h. Signals were visualized with Super Signal West Pico Chemiluminescent Substrate Kit (Pierce). Band intensities were analyzed by GelPro gel analysis software (BioRad). β-actin or GADPH was used as reference for the experimental data analysis.
Stastistical analysis
All experiments were repeated at least three times. Data were performed by GraphPad Prism version 5.0(GraphPad Software Inc., San Diego, CA) and SPSS 17.0 statistical software package (SPSS, IL, USA) and reported as mean±SD. The differences were determined by using the Student’s t-test and chi-square test. P<0.05 was determined to be statistical significance.
Intrinsic cytotoxicity of DHA
To determine the optimal dose of DHA, we performed a MTT assay to access the viability of A2780 and A2780/T against DHA treated for 48h (Figure 1). At the concentration of 64µM/L, DHA had insignificant effect to the A2780, while the maximum safe dosage for DHA in A2780/T was 296um/L (survival rates of cancer cells were more than 90%). Due to the low growth-inhibiting effects, treatment of DHA at 64µM/L and 296µM/L was selected in subsequent experiments.
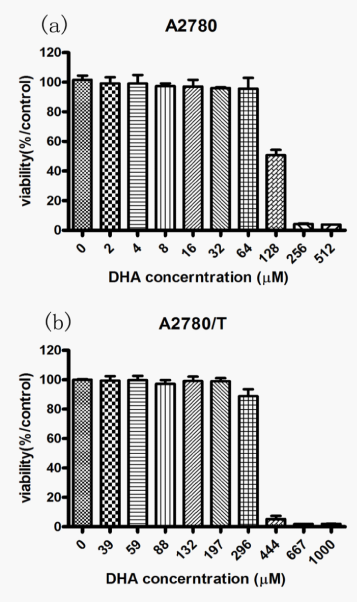
DHA reversed taxol resistance in A2780/T cells
A2780 and A2780/T cells were pretreated in DHA (64µM, 296µM respectively) for 48h combining with various concentrations of Taxol (0-32µM). As shown in Table 1, DHA significantly enhanced the cancer cells sensitivity to Taxol in A2780/T cell line. The IC50 value of A2780/T cells to expose Taxol alone or in combination with DHA were 5.42+1.451µM/L and 1.83+0.532µM/L respectively, which exhibited a greater reversal to Taxol (2.96-fold). However, as shown in the Figure 2A and Table 1, DHA had almost no effects on the Taxol toxicity of A2780. The result indicated that DHA reversed Taxol resistance in A2780/T cells rather than sensitive cells.
|
Cell |
N |
IC50 (mean±SD, μg/ml) |
RI |
|
A2780 |
3 |
0.0463±0.00725 |
1.038 |
|
A2780+DHA64μM |
3 |
0.0446±0.005667 |
|
|
A2780/T |
3 |
5.42±1.451 |
2.96 |
|
A2780/T+DHA296μM |
3 |
1.83±0.532* |
Table 1 Drug sensitivity of A2780 and A2780/T

DHA enhanced the accumulation of rhodamine123 in A2780/T cells
To determine whether DHA caused an increase on the intracellular taxol accumulation, the Rhodamine123 in presence or absence of DHA was examined by FCM analysis. Figure 3A & 3B showed that intracellular Rhodamine123 in A2780 cells was greatly higher than it was in A2780/T.As shown in the Figure 3 and Table 2, after 48h pretreatment of DHA with different dosage (11-296μM), intracellular Rhodamine123 accumulated in a dose-dependent manner. The higher dosage of DHA pretreated, the more Rhodamine123 accumulated in A2780/T cells, the data suggested that DHA could promote the chemotherapeutic drug accumulation to exert the cytotoxic effects in a concentration dependent manner.
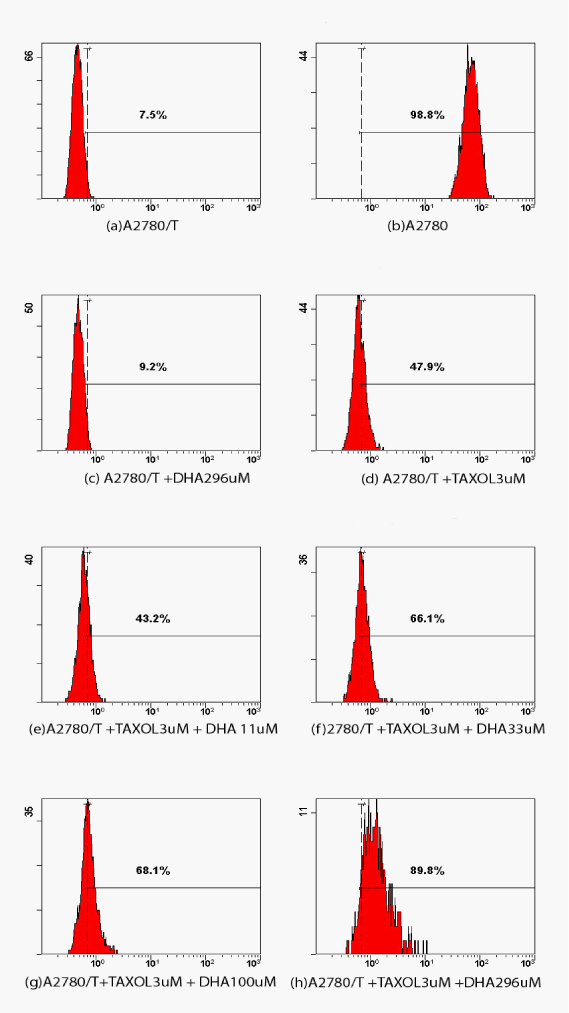
|
Number |
Groups |
Accumulation of rhodamine123 in cells |
|
A |
A2780/T |
7.50% |
|
B |
A2780 |
98.80%* |
|
C |
A2780/T+Taxol3uM |
47.9%%* |
|
D |
A2780/T+DHA300μM |
9.2%* |
|
E |
A2780/T+Taxol3μM+DHA11μM |
43.2%* |
|
F |
A2780/T+Taxol3μM+DHA33μM |
66.1%* |
|
G |
A2780/T+Taxol3μM+DHA100μM |
68.1%* |
|
H |
A2780/T+Taxol3μM+DHA296μM |
89.8%* |
Table 2 Accumulation of rhodamine123 in cells compared with A2780/T
*P<0.001
DHA arrested cell cycle progression in A2780/T cells
As shown in Table 3, after 104 cancer cells were treated with Taxol 3μM and different doses of DHA, the proportion of A2780/T cells in G0/G1, were 4.5%, 5.7%, 18.4% and 20.6% respectively. Meanwhile, with increasing concentration of DHA, the proportion of G0/G1 was increased, but the proportion of cells in M/G2 was decreased (84.1%, 84.9%, 75.1%, and 64.3% respectively) significantly. Those indicated that DHA in combination with Taxol could alter the cell cycle distribution and arrest the cell cycle in G0/G1 phase.
|
|
G0/G1 |
S |
M/G2 |
c2 |
P |
|
Taxol 3μM |
453(4.5) |
1137(11.4) |
8410(84.1) |
2058.17 |
<0.001 |
|
Taxol 3μM+DHA 33μM |
570(5.7)** |
937(9.4) |
8493(84.9) |
||
|
Taxol 3μM+DHA 100μM |
1840(18.4)** |
1390(13.9) |
7510(75.1)** |
||
|
Taxol 3μM+DHA 296μM |
2057(20.6)** |
1517(15.2) |
6426(64.3)** |
Table 3 Cell cycle distribution of A2780/T cells (cell n (n%)). Compared with taxol 3μM
**P<0.001
DHA down-regulated MDR1 mRNA and MDR-related proteins expression
MDR phenomenon is associated with MDR-related proteins, including MDR1, MRP, BCRP and LRP. In our previous studies, MDR-related proteins were significantly increased in A2780/T cells. Quantitative RT-PCR and Western Blot were performed to detect the change on MDR1 mRNA and MDR-related proteins with the treatment of DHA in A2780/T cells. As shown in Figure 4C, compared with control group (without DHA), MDR1 mRNA was obviously decreased in the group of DHA. Figure 4A & 4B showed that the expressions of MDR1, MRP1, MRP2, BCRP AND LRP proteins were significantly decreased after treated by DHA. In addition, compared with Taxol alone, combination with DHA markedly decreased those proteins level obviously, which indicated that DHA could act synergistically with Taxol and the reversal effects might be associated with the suppression on the MDR-related molecules, such as MDR1, MRP1, MRP2, etc.
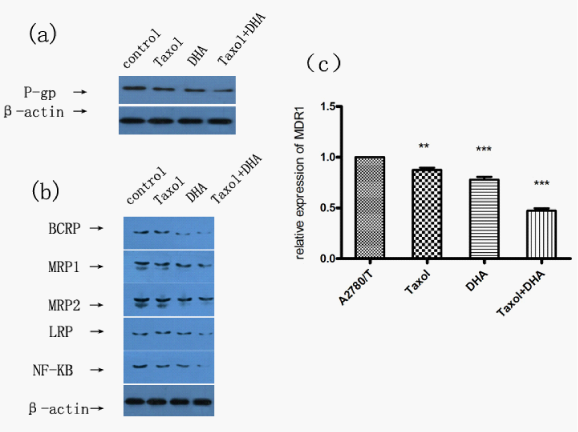
DHA inhibited pp38MAPK and NF-κB activity
Based on the reversed Taxol resistance of DHA in A2780/T by down-regulating the mRNA and MDR-related proteins, we speculated that some signaling pathways might be connected with the effects of DHA on drug resistance. To further investigate the mechanisms underlying reversal effects of DHA, Western Blot was performed to detect the levels of p38MAPK, PP38MAPK and NF-κB in A2780/T cells. As shown in Figure 5, pp38MAPK and NF-κB were conformed suppressed in A2780/T cells pretreated with DHA and Taxol, whereas no significant changes in total p38MAPK expression were noted in the experiments, which suggested that combination treatment of DHA and Taxol could dramatically suppress pp38MAPK and NF-κB expression. Considered together, our results indicated that DHA reversed Taxol resistance by inhibiting the function and expression of MDR-related proteins and via suppression the activation of NF-κB and phosphorylation of P38MAPK.
Ovarian cancer ranks first in mortality among women in the world.1 When ovarian cancer occurs, survival length and quality of life rely heavily on the sensitivity to anticancer drugs. MDR is the primary cause of chemotherapeutic failure ultimately and the most life-threatening in ovarian cancer patients. Consequently, MDR reversal has become a current focus for overcoming barriers in the treatment of ovarian cancer. The study was performed on DHA to explore its role on reversing drug resistance and the underlying mechanisms.
N-3 polyunsaturated fatty acids (n-3 PUFAs), naturally contained in algal and fish oils, have been reported to play a vital role in carcinogenesis and cancer outcomes.11,12 Serious studies have shown that DHA and EPA as members of n-3 PUFAs could give rise to MDR reversal in cancer cells. MDR is the ability of tumor cells to develop resistance to multiple classes of chemotherapeutic drugs that may be structurally diverse and mechanistically different. As reported by Plumb,13–15 PUFAs could enhance doxorubicin cytotoxicity to ovarian cancer cells. Meanwhile, it also increased the arsenic trioxide cytotoxicity to SCOV-3 cells, and could help to overcome the natural and acquired resistance to arsenic trioxide.14 Intending to study the reversal effect of DHA and EPA on ovarian cancer cells, we established a cell line of Taxol resistance by stepwise sequential exposure to increased concentration of Taxol.15 The drug resistance index (RI) was 430.7. Then, we performed MTT assays to access the change of Taxol toxicity against A2780/T with DHA or EPA for 48h in our preliminary experiments. As expected, the results showed that DHA dramatically enhanced the sensitivity of cells to Taxol. However, EPA had no such effects on the cytotoxicity of Taxol against A2780/T (data was not shown). Hence, it was reasonable to make a conclusion that DHA might reverse Taxol resistance in A2780/T cells. In addition to A2780/T, we also perform a parallel test in A2780. Whereas, it was worthy to note that reversal effect of DHA were faintly in sensitive cells.
In clinic, recurrence of the initially sensitive cells was involved in acquired drug-resistance, including a higher capacity to progress through cell cycle in response to chemotherapy. Our preview researches were associated with biological characterizes of drug resistance cells. Due to the non-cycling dormant cells, A2780/T grew slower than parental cells A2780. We found that the percentage of GO/G1 in A2780/T cells was higher than in A2780 and the S phase was lower. Focusing on the changes in cell cycle, we next examined it in A2780/T cells pretreated of DHA with different dosages. Cell cycle analysis showed a markedly increasing in G0/G1 with the reducing proportion of cells in M/G2 in a dose-dependent manner. As a result, DHA in combination could alter the cell cycle distribution and increase the percentage of non-cycling dormant cells.
Besides MTT assay, we also performed Rhodamine123 accumulation assay to evaluate the role of DHA on reversing drug resistance. The data showed that after pretreatment of DHA, the intracellular Rhodamine123 concentration increased significantly in a dose-dependent manner. The results suggested that DHA could promote the chemotherapeutic drug accumulation to exert the cytotoxic effects in a concentration dependent manner.
As is known to all, the intracellular Rhodamine123 concentrations in MDR cells were extremely lower than sensitive cells. Meanwhile, the molecular mechanism of MDR is not fully clarification. It is well known that formation of MDR is complicated process and involves multiple factors, including increased drug efflux, alteration the apoptotic response, and change of DNA damage repair capacity in drug resistance tumor cells.16 Among these factors, the most important reason is the drug efflux resulting from enhancement of drug transporter activity. These transporters originated from ATP-binding cassette (ABC) transporters super family such as P-gp, MRP1, MRP2, BCRP and LRP.17 Accumulated evidences have indicated that over expression of P-gp directly contributes to MDR, and blocking P-gp drug pump functions and suppressing its expression might enhance the effects of chemotherapeutic drugs on MDR cancer cells.18 Giada has reported that they reverse MDR in colon cancer cells by inhibiting the expression and function of P-gp.19 To define the underlying mechanisms, we used Western Blot to detect the effects of DHA on P-gp expression in A2780/T cells. After 48h pretreatment of DHA, the expressions of P-gp were decreased. Due to the faint effects on sensitive cells, we speculated that DHA reversed Taxol resistance in A2780/T by mediating over-expression P-gp. To date, some designed compounds of P-gp inhibitors have been developed to increase the susceptibility of cancer cells to chemotherapy.18 Despite having reversal functions, most of these agents may cause serious side-effects on necessitated high doses. In this case, natural supplement agents might have the advantages in clinic in future.
Although the underlying mechanism of DHA on MDR reversal remained unclear, a great number of studies have reported the mechanism of other reversal agents. Among of them, the abnormal activation of some signaling pathways were related with MDR, including mitogen-activated protein kinase (MAPK) cascade and phosphatidylinositol-3-kinase (PI3K)/Akt signaling pathway.20–22 Zhao suggested that Grape Seed Procyanidin reversed MDR via down-regulation of NF-κB and MAPK signaling.23 Vinorelbine could increase cytotoxicity of cisplatin to human lung cancer cells by inhibiting the activation of NF-κB.24 NF-κB, composed of P65 and P50, relies on the inhibitory molecules of IκB (inhibitor of NF-κB) family. Phosphorylation of submit and degradation of IκB promote NF-κB to translocate from the cytosol to the nucleus. It has been identified NF-κB binding sides in MDR1 promoter region.25 Therefore, NF-κB pathway was found related with developing multidrug resistance in several cancer cells.23,24,26
Meanwhile, P38MAPK has been reported to serve as an upstream NF-κB regulatory kinase.27 p38-MAPK, an important member in MAPK family, is closely correlated with the effects of chemotherapeutic drugs, including chemotherapeutics against ovarian cancer.28 Previous studies have reported that MAPK activity was positive correlation with MDR1 and involved in the regulation of P-gp expression.29,30 It was said that loss of the ability to activate p38 might be a general mechanism involved in the acquisition of chemo-resistance.31 Zhang suggested that knocking-down Annexin A2 might reverse drug resistance in SGC7901/DDP by regulating P38MAPK pathway.32 Moreover, p38MAPK signal inhibitors, SB203580, were demonstrated as a reversal agent of P-gp mediated multidrug resistance.26 Furthermore, in ovarian cancer cells, Villedieu found that chemo-resistance was accompanied with p38 activation.33 Hence, it was reasonable to speculate that DHA might reverse Taxol resistance in A2780/T cells by inhibiting NF-κB and p38-MAPK pathway.
Our data showed that NF-κB signaling and pp38MAPK were markedly inhibited by DHA. However, we did not note significant changes of total p38MAPK expression in the experiment. The results directly implied that DHA suppressed the phosphorylation of P38MAPK rather than p38MAPK, and made the hypothesis certain. Taking into account the relationship among P-gp, P38MAPK and NF-κB, we suspected that P38MAPK might regulate P-gp expression through the transcription factor, and the reversal effects of DHA on Taxol resistance might be in the same manner.
To conclude, as the results shown in our study, DHA might have beneficial effects on reversing drug resistance. The effects of DHA on ovarian cancer were linked to inhibiting the function and expression of MDR-related proteins and suppressing the activation of NF-κB and phosphorylation of P38MAPK. Further investigations were needed to assess the broader benefits and underlying mechanism of DHA in the fight against cancer. There is no doubt that intravenous DHA infusions that is being supplemented method will be widely used in future.
This work was supported by grants from National Science Foundation (81473636), the Chinese postdoctoral support program (2013M542577), Jiangsu Government Scholarship “333” Plan and Jiangsu Key Medical Personnel (RC2011091).
The authors declare there is no conflict of interest.

©2014 Wu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.