eISSN: 2377-4304


Research Article Volume 11 Issue 3
1Department of Oncology andDepartment of Biomedical and Clinical Sciences, Linköping University, Sweden
2Department of Clinical Chemistry, Department of Biomedical and Clinical Sciences, Linköping University, Sweden
3Regional Cancer Center South East Sweden and Department of Biomedical and Clinical Sciences, Linköping University, Sweden
Correspondence: Ann-Lisbeth Liest MD, Department of Medical Oncology, County Council of Ostergotland, Linkoping University, S-58185 Linkoping, Sweden, Tel 46-705291193, Fax 46-1031417
Received: April 30, 2020 | Published: June 8, 2020
Citation: Liest AL, Omran AS, Mikiver R, et al. Prospective study of the role of HE4 and CA125 in treatment and follow-up in ovarian cancer patients. Obstet Gynecol Int J. 2020;11(3):185-190. DOI: 10.15406/ogij.2020.11.00507
Introduction: Epithelial Ovarian Cancer, tubal- and peritoneal cancer (EOC) is still the major cause of death in gynecological cancer. The outcome of primary surgery is an important prognostic factor. The primary aim of this study was to study the utility of HE4 and CA125 in monitoring the response of chemotherapy during treatment and in predicting prognosis and recurrence during follow-up. We have also evaluated the role of HE4 as a predictor of the result of surgical intervention.
Methods: Seventy-eight patients scheduled for chemotherapy were monitored with serum HE4 and CA125 during treatment and follow-up. In 39 patients samples for tumor markers were also obtained prior to surgical intervention.
Results: Both HE4 and CA125 decreased in response to treatment. PFS and OS were strongly dependent on HE4 levels prior to start of chemotherapy with significantly prolonged PFS and OS when HE4 levels were under upper reference limit of 82pmol/L (P=0.018 resp. P<0.001).
The levels of HE4 correlated with primary surgical outcome with significantly lower postoperative HE4 in the radically operated group (P<0.001). An increase in HE4 and/or CA125, signals a recurrence 3-6months before diagnosis. Median levels for both HE4 and AC125 before start, during and at the end of treatment were significantly higher for platinum resistant patients (P<0.005).
Conclusion: Both tumor markers are valuable in monitoring the response of chemotherapy as well as in predicting recurrence during follow-up. Postoperative HE4 holds promise as an objective marker to evaluate the result of surgery and is highly predictive for future prognosis.
Keywords: ovarian cancer, prognosis, HE4, CA125
HE4, human epididymal protein 4; CA125, cancer associated antigen 125; PDS, primary debulking surgery; NACT, neo adjuvant chemo therapy; PFS, Progression Free survival; OS, overall survival; URL, upper reference limit
Epithelial Ovarian Cancer, tubal- and peritoneal cancer (EOC) is still the major cause of death in gynecological cancer despite improved chemotherapy and more recently introduction of new biological therapies. The outcome of primary surgery conducted with the intention to achieve complete cytoreduction is one of the main prognostic factors.1 Patients presenting with a malignancy suspected to be originating from ovaries, fallopian tubes or peritoneum should be subjected either to primary debulking surgery or neoadjuvant chemotherapy (NACT) as initial treatment. However, since more than 60% are in advanced stage at diagnosis2 complete primary cytoreduction is not always feasible. Neoadjuvant chemotherapy followed by delayed surgery has been proposed to result in the same clinical outcome as primary surgery in combination with post-operative chemotherapy when complete initial debulking is not deemed to be possible.3,4 The decision to initiate debulking surgery is based on imaging diagnostics in combination with clinical and laboratory findings, performance status and comorbidity.3 Serum levels of tumor marker CA125 are most often elevated, but have no established role in the decision regarding subsequent management. Delayed surgery, with the goal to achieve complete tumor debunking, should be considered after 3-4 cycles of NACT. In this context, a decrease of CA125 during NACT is an important factor.
The tumor marker HE4 (Human Epididymis Protein) is used in the ROMA-algoritm (Risk of Ovarian Malignancy Algoritm) to distinguish a malignant pelvic mass from benign disorders.5 HE4 has also been evaluated as a tool to predict the possibility of achieving optimal surgical debulking, for monitoring the effect of chemotherapy and for predicting prognosis.6–11
The primary aim of this study was to study the utility of HE4 and CA125 in monitoring the response of chemotherapy during treatment as well as in predicting prognosis and recurrence during follow-up. Secondary aim was to evaluate the role of HE4 as a predictor of the result of surgical intervention.
This is a prospective, non-randomized multicenter study comprising patients from the referral area of the Department of Oncology, gynecologic section, University Hospital in Linköping, Sweden. The majority of the patients underwent surgery at the Department of Gynecology, University Hospital in Linköping, Sweden, but received chemotherapy at their referral hospitals in accordance with a treatment plan formulated by gynecologic oncologists at the University Hospital.
Women ≥18years of age diagnosed with ovarian cancer and planned for NACT or post-operative chemotherapy were included. Patients were recruited when they received information about the planned treatment. Informed consent was obtained from all patients. A total of 78 patients with EOC were included between November 2013 and April 2016.The first 39 patients participated in a study where samples for tumor markers were obtained prior to surgical intervention. The next 39 patients were first included after surgery or when NACT was initiated, which unfortunately resulted in a smaller number of participants with both pre- and postoperative marker values.
The patient group comprised predominantly of postmenopausal patients (n=71) with median age 67years. Only seven, median age 49years were premenopausal. All patients were followed from start of chemotherapy up to 5 years, relapse or end of follow-up for other reasons. Blood samples for analysis of HE4 and CA125 were obtained at the days of chemotherapy. We also obtained samples within one week prior to surgery in 33 patients. Blood samples for analysis of tumor markers were also taken at each follow-up visit. The laboratory results were blinded for both clinicians and patients.
All patients were treated with Paclitaxel 175m2 and Carboplatin AUC 5 combinations q3w aiming for 6 courses with the exception of three patients, who due to age and performance state received Carboplatin only. Reduction of doses, switch to other regimes as well as follow-up with 3-6 months interval was according to the national guidelines.
Analysis
All samples were transported at -20˚C and stored at -80˚C pending analysis.
All CA125 and HE4 assays were performed at the Department of Clinical Chemistry, University Hospital, Linköping, Sweden to avoid inter-laboratory variability. Both CA125 and HE4 were measured by an electro-chemiluminescent immunoassay (ECLIA) on the automated Cobas e602 (Roche Diagnostics GmbH, D-68305 Mannheim, Germany). The CA125 method has been standardized against the Enzymun Test CA125 II method, which in turn has been standardized against the CA125 II RIA from Fujirebio Diagnostics. HE4 method has been standardized against the HE4 EIA method from Fujirebio Diagnostics, Inc. (Gothenburg, Sweden).
Statistical analysis
Difference between HE4- and CA125 expressions at different times and other variables were evaluated using Wilcoxon Signed Ranks Test and Mann-Whitney Test when appropriate. Survival curves were created using the Kaplan–Meier method, and statistical significance was determined by Log Rank (Mantel-Cox) test. For all statistical analysis, P value of less than 0.05 was regarded as statistically significant. All statistical analyses were done with SPSS (version 25). The sample size was not sufficient to perform a multivariate analysis.
Ethics
The study was approved by the local committee of ethics (Ref. 2012/141-31, 2013/424-31, 2014/330-32, 2016/121/3, 2018/64-32).
Patient characteristics
Seventy-eight patients were included. One cohort of 39 patients was subjected to primary debulking surgery. Blood samples for analysis of tumor markers were obtained from 33 due to logistical mishaps in six cases. A second cohort of 39 patients had recorded tumor markers from the start of chemotherapy only. In this second group 23 patients underwent primary debulking surgery. Hence, in total, 62 patients underwent primary surgery. Complete debulking was achieved in 47 patients (75.8%) out of these 62 patients. Sixteen patients commenced treatment with NACT followed by delayed surgical intervention in 12 cases. Complete cytoreduction was possible in only 4 of these (33.3%). Four patients were not subjected to any surgical intervention at all.
The majority of patients 71 (91%) were postmenopausal and seven (9%) were premenopausal. All 27 patients diagnosed in early stages (FIGO I and II) were subjected to primary surgery, as were 35 of patients in advanced stages (FIGO III and IV). Sixteen patients with advanced disease started their treatment with chemotherapy. As expected the main histopathological types (86%) were high-grade serous, clear cell and endometroid cancers. Low grade serous were found in 8% of the patients and 6% had serous non-graded cancers.
Levels of HE4 and CA125
In the cohort of 33patients with recorded values of both pre- and postoperative tumor markers the median levels of HE4 significantly decreased to well under upper reference limit (URL) in the group in which complete debulking was achieved (n=28) compared to the group (n=5) left with minimal or bulky disease (P<0.001, Figures 1A–1B). In the present study, we have used 82pmol/L as the URL in accordance with conclusions from our previous study.12 The four patients left with bulky disease had postoperative HE4 values, which were well above the URL. Similarly, patients with no macroscopic tumor postoperatively had significantly lower median postoperative CA125 values compared to those left with minimal or bulky disease (P=0.002, Fig.1 C-D). Eight patients (22%) reported as radically operated had HE4 levels above URL postoperatively whereas 16 of 36 patients (44%) still had CA125 levels above URL postoperatively. This difference persisted even when we combined both cohorts. In the whole material of 74 patients undergoing surgery 13 (17%) had HE4 above URL and 28 (38%) CA125 above URL when completely debulking surgery was reported.
There were no significant difference betweenmedian preoperative levels of neither HE4 nor CA125 in the groups with radical surgery versus non-radical surgery.
Treatment
In 6 out of 78 patients the planned sampling of tumor markers before the start of chemotherapy were not obtained due to logistical issues. Elevated HE4 levels decreased significantly between cycle 1 and 6 (P<0.001). However normal HE4 levels at the start of chemotherapy did not decrease any further. Similarly CA125 levels above URL decreased significantly during treatment (P<0.001).
Progression free survival (PFS) and overall survival (OS)
Progression free survival (PFS) was strongly dependent on the levels of HE4 prior to start of chemotherapy (Figure 2A). PFS was significantly prolonged if HE4 levels were below URL compared to patients with elevated HE4 levels at the start of treatment (P=0.018). A pathologically elevated HE4 at cycle 1indicated a shorter PFS even if the value normalized before cycle 6 (P=0.012). Even OS is dependent on HE4 levels prior to start of chemotherapy with significantly prolonged OS (P<0.001) in patients with HE4 values below URL. We also found that OS improved significantly (P<0.05) even when HE4 values were above URL prior to start of chemotherapy but normalized at cycle 6 (Figure 2B). In these results patients achieving NACT are included. All these patients had high levels of both tumor markers. Likewise, a normal CA125 at cycle 1 indicated a significantly longer PFS compared to patients with elevated CA125 prior to chemotherapy (P=0.017, Figure 2C). However, neither PFS nor OS were significantly prolonged in patients with elevated CA125 levels at cycle 1 but normalized tumor marker at cycle 6 when compared to patients with CA125 above URL at the end of chemotherapy (Figures 2C–2D).
Relapse
The median follow-up was 35 months (range 0-68). Relapse was defined as CT verified signs of new tumor lesions in patients with no earlier evidence of disease. Both CA125 and HE4 most often reacted similarly with coinciding elevations in the 21 patients with relapse. An increase in HE4 levels preceded an increase in CA125 values in only two cases. The interval from first noticed increase of tumor markers above URL to a verified recurrence was between 2-6 months with a majority having a recurrence between 3-4 months (Figure 3).
Platinum resistance
In our material, 10 of 78 patients showed platinum resistance, defined as relapse less than 6 months after the last chemotherapy treatment or remaining tumor after platinum based chemotherapy. Median levels of both HE4 and CA125 before start, during and at the end of treatment were significantly higher (P<0.005) in platinum resistant patients (Figure 4). No significant differences were observed median HE4 levels at the start of treatment, after three cycles and at the end of treatment in the platinum sensitive group. In patients with platinum resistant disease the median HE4 levels dropped during treatment from 651 to143pmol/L after three cycles and to102pmol/L at cycle 6. All those patients had remaining elevation of CA125 at cycle 6. In platinum sensitive patients the median CA125 level were over URL at the start of treatment but normalized after three cycles.
Several authors have addressed the role of HE4 during treatment and follow-up. The preoperative level of HE4 has been advocated to be a predictor of the possibility of performing radical debulking surgery with reported cut-off values varying between 154-262pmol/L.7,10,13,14 A preoperative level of HE4 above a median level of 394pmol/L has been reported to be significantly associated with progression and mortality.15 We could not corroborate these results as we found no significant differences in preoperative levels of neither HE4 nor CA125 in the group that was radically debulked compared to the group left with macroscopic disease. Our lack of corroboration could partly be due to limitations in our sample size with only a small number of patients with both pre- and postoperative tumor markers samples.
The result of the surgical intervention is assessed by the surgeon at the end of operation and currently there are no objective variables indicating radicality. It is therefore interesting to note that we found significantly lower median levels of HE4 and CA125 in patients where complete cytoreduction was achieved compared to the group left with minimal or bulky disease. Although CA125 also decreased after a successful cytoreduction, a majority of these patients still had elevated CA125 values. The levels of CA125 may be elevated postoperatively due to a peritoneal reaction after the surgical procedure. Hence, a postoperative elevation of CA125 has to be considered in relation to the time between surgery and blood sampling as well as the clinical condition of the patient. The median time from surgery to start of chemotherapy in our study was 42 days (range 13-92). It would therefore seem reasonable to conclude that surgical trauma has no impact on the CA125 levels prior to start of chemotherapy. However, as HE4 is reported to be independent of peritoneal reactions,16 and as the half time clearance is less than 4 hours, we would like to postulate that a normalized HE4 value postoperatively is a promising marker to evaluate the result of surgery and merits further investigation.
Chemotherapy, PFS and OS
The role of HE4 in monitoring chemotherapy has been evaluated regarding prognosis and prediction of platinum resistance. The serum levels of HE4 were under URL in 86% of patients reported as optimally tumor reduced. The median levels of HE4 in patients with normal values at the start of chemotherapy remained below URL during treatment whereas elevated HE4 levels at start of treatment decreased significantly. A similar result was seen for CA125 (Fig.5). Based on our data, we suggest that HE4 and CA125 are valid markers to monitor the response to chemotherapy, but only when the markers are above the normal range prior to start of chemotherapy. As a majority of patients (68 %) had elevated CA125 levels when treatment was started and only 42% had elevated HE4 levels, the role of CA125 as the most important marker for monitoring chemotherapy is thereby further confirmed and in line with Ferraro.18
We found significantly prolonged PFS and OS in patients with HE4 levels under URL 82pmol/L at the start of chemotherapy. Further, PFS was significantly shorter even when HE4 values were above the URL at start of chemotherapy but dropped to normal range after three and six cycles of chemotherapy. These results are in concordance with Steffensen et al.6 who found a significant shorter PFS in 139 patients with high levels of HE4 just before start of chemotherapy. Also, Kong et al.9 described that the pretreatment HE4 level was a predictor of prognosis. On the other hand, in our study the level of CA125 was not predictive of either PFS or OS. Our results are in contrast to Rocconi et al.,19 who showed that both PFS and OS were significantly longer if CA125 was normalized after three cycles of chemotherapy regardless of the initial value.
Relapse and platinum resistance
It is well known that the majority of patients treated for advanced EOC will have a relapse within few years after end of treatment. Definition of recurrence in the case of CA125 is as defined by the Gynecologic Cancer Intergroup.20
The question about monitoring CA125 levels after adjuvant treatment for EOS in patients in complete remission has been intensively debated.21–24 Diagnosis of recurrent disease is often preceded by an increase of CA125, roughly two to six months before clinical symptoms, raising the possibility of starting chemotherapy based solely on increasing CA125 levels.
Rustin et al.22 demonstrated no improved survival but decreased quality of life in a group with early re-treatment compared to a group with start of treatment first after subjective symptoms. His study has been subject to criticism as the re-treatment regimens were very diverse and no patient underwent surgical intervention. On the other hand Fleming et al.25 showed that delayed re-treatment, after an obvious increase in CA125 levels, decreased the possibility of achieving optimal tumor resection by a second surgical effort. To monitor and react on a rise in tumor markers could be further advocated by the DESKTOP III study that concluded that surgery in a situation of relapse affected prognosis but only when complete resection of all macroscopic tumor lesions could be attained and only in platinum-sensitive patients.26
Reports concerning the role of HE4 during follow-up have suggested that HE4 is a more sensitive marker for diagnosis of recurrent EOC than CA125.27,28 Most of these studies are small, but Steffensen et al.11 demonstrated in a material of 88 patients that HE4 is a highly sensitive marker for relapsed disease. Anastasi et al.29 observed an earlier increase of HE4 than CA125 in a very small group of eight patients with recurrence. In our material 21 recurrences were observed during the observation time of up to 68 months. Unfortunately, we did not succeed in obtaining samples from all planned visits as many dropped out due to practical difficulties. Despite this we can still conclude that both HE4 or CA125 alone or even better both together, indicate a recurrence with elevations occurring most often in parallel, roughly three to four months before confirmed recurrence (Figure 5). Further research is needed to address the implications of increasing tumor markers. It has been suggested that HE4 levels are a predictor of platinum resistance.30,31
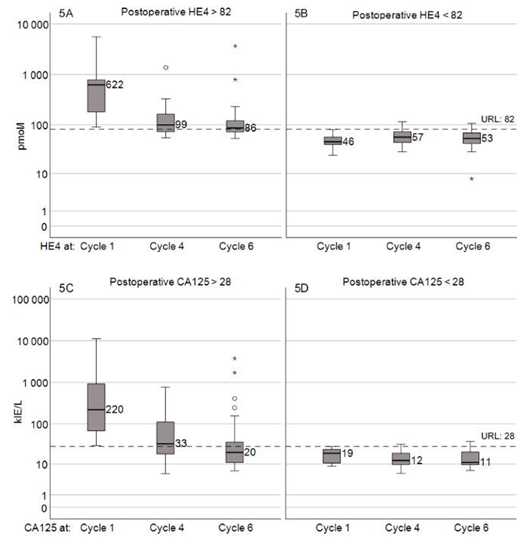
Figure 5 Tumor markers during chemotherapy in patients that underwent all 6 cycles of chemotherapy. HE4 in total 54 patients (C1>82: 24, C1<82: 28). CA125 in total 51 patients (C1>28: 36, C1<28: 15).
Due to limited number of patients we have not investigated the role of HE4 in predicting the possibility of performing complete tumor resection after NACT. This is important for prognosis as a surgical intervention without complete tumor resection is of no benefit for the patient and in addition the interval to next chemotherapy might be prolonged. HE4 has been described as a useful tool in this question.27,32
In summary we can conclude that the levels of tumor markers at start of chemotherapy seem to be the most important factors for PFS and OS and also a predictor of platinum resistance. HE4 does not signal a recurrence earlier than CA125. We find the role of HE4 after surgical intervention to be of importance and suggest future studies of HE4 levels before and shortly after primary surgery for an objective evaluation of the surgical outcome and thereby also as a predictor of prognosis.
We would like to thank Christina Jederud, responsible study-nurse for the project during initialization and first years of the study and Pernilla Nilsson, responsible study-nurse during the last years. Special thanks to all principal investigators, gynecologists and nurses from the recruiting centers in Linköping, Norrköping, Kalmar, Västervik, Jönköping, Värnamo, Eksjö, Nyköping and Västerås. Thanks also to SQRGC, Swedish Quality Registry of Gynecological Cancer.
None.
The authors report no conflicts of interest.

©2020 Liest, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.