eISSN: 2377-4304


Review Article Volume 14 Issue 1
Ministry of Higher Education, Iraq
Correspondence: Saadi JS AlJadir, MB ChB, PhD, MRCP, FACE, FRCP (L), FRCP (G), Ministry of Higher Education, AlMansour, AlMutanabi, District 607, Alley 20, House 9, Baghdad, Iraq, Tel +9647813902926
Received: January 03, 2023 | Published: January 16, 2023
Citation: AlJadir S. Probiotics and women health: clinical perspective. Obstet Gynecol Int J. 2023;14(1):1-9. DOI: 10.15406/ogij.2023.14.00684
Fermentation of foods had been dated to early human life on the planet far more before civilization. The transition from hunting and gathering to the agricultural lifestyle might have triggered task of food fermentations, nowadays this task is carried on industrial level. Many parts of the human body; the skin, oral cavity, gut, and vaginal canal are populated by huge numbers of microbes.
At birth, human gut is a sterile environment, however it will start to be colonized instantly after birth. Factors such as diet (formula or breast feeding) and type of delivery (either vaginal birth or abdominal) can both impact the colonization patterns. The pioneer microbes inhabiting the gut make permanent adaptations and thus determining the metabolic, physiological, behavioral, and immune development which will encourage vulnerability to diseases. Because Age and lifestyle are associated with alteration in microflora, therefore are of some causes of diseases. Latest research has shown that microbiota composition is remarkably different in diseases such as obesity and periodontal diseases with healthy individuals usually showing diverse, distinct, and temporary stable microbiota communities at these sites in comparison with individuals manifesting disease.
Keywords: probiotics, fermentation, women health, human life, civilization
FAO, food and agriculture organization of the United Nations; WHO, World Health Organization
Gut microbiota has received tremendous interest and attention especially for last two decades. It is considered by the scientists in this field an important player in various metabolic aspects and that might have an impact to diseases in humans.
Moreover, it can behave as an endocrine organ, metabolizing nutrients in human diet and generating variety of metabolites. These metabolites can actively act after being absorbed the intestine’s receptors and extra intestinal organs, liver, adipose tissues, immune system, muscles, and brain. Metabolic diseases such as T2 Diabetes, obesity, cardiovascular disease, and liver steatosis, all have been shown to be modified by microbial products and their metabolites. There is sufficient evidence which supports a causal relation of gut microbiota in the development and progression of these common and increasing disorders.
We have reviewed some of the aspects about how microbiota is evolved and operates as a complex microbial ecosystem, and the various alterations in the microflora composition detected in different metabolic diseases. It also shades some light on the metabolic effects of microbial products, and therapeutic potential of addressing the gut and vaginal microbiota for management of common health problems in women.
Necessary definitions
Probiotics are either the following: microbial cell preparations, or their components or particles that might have a beneficial impact on human health. Probiotics are either viable or non-viable both have shown positive health benefit. On the other hand, metabolites are, not included in the current definition, therefore, it excludes antibiotics.
The suggested definition is based upon the mechanisms of action, viability and non-viability, selection criteria, and scientifically supported health effects that will be mentioned.
Microbiome: is the collective microorganisms’ genomes in a specific environment.
Microbiota: the community of microorganisms.
There are approximately 100 trillion microorganisms (vast are bacteria, but also viruses, protozoa, and fungi) present in the human gut. The best depiction of a microbiome in the human body is as a virtual organ of that body.
Human genome comprises of about 23,000 genes; however, the microbiota comprises of over 3 million genes, producing large numbers of metabolites, which affect directly or indirectly the various functions in the host’s body, and eventually influencing the host’s health, immunity, and her (or his) phenotype.
5 facts about probiotics
Probiotics are living microorganisms of about 90% bacteria and are either the same as or like microorganisms found naturally in the human body and may offer some benefits to overall human health. If we can perform an imaging to the human body (as a host) for bacteria and microbes (viruses, fungi, or protozoa), we might obtain a sound understanding of probiotics. The gut (especially the lower GI) holds a diverse and complex community of bacteria. Although we used to believe that bacteria are harmful microbes, many bacteria (with other microbes) are of remarkable benefits and assist the human body to operate properly.
Probiotics are available to individuals in the form of oral products such as dietary supplements, suppositories, and creams and even foods (e.g., yogurt). It is vital to know that the FDA has not approved any health supplements for probiotics!
Here are some facts for your awareness:
History
The word probiotic is (pro means in Latin for and Bios in Greek life) for life, was firstly presented by German scientist Werner Kollath in 1953 to describe “Active substances that are essential for a healthy development of life.”
In the mid-60s, this term was utilized by Lilly and Stillwell in a different notion to describe “substances secreted by one organism which stimulate the growth of another.” In 1992, Fuller described probiotics as “a live microbial feed supplement which beneficially affects the host animal by improving its intestinal microbial balance.”
Modern history of probiotics had started at the beginning the 20th century with the innovative research of the Russian scientist Elie Metchnikoff (Nobel laureate), who had worked at the Pasteur Institute in Paris. Pasteur has identified the microorganisms in charge of the fermentation process, while Metchnikoff tried first to find out the possible effect of these microbes on human health. He attributed the longer life the rural Bulgarian’s community had entertained, to their indulgence in enjoying fermented dairy products (e.g., yogurt). He associated this to the Bulgarian bacillus which was recently discovered by a young Bulgarian physician Stamen Grigorov. Afterwards, he proposed that lactobacilli might antagonize the putrefacient effects of gut metabolism that were main factors to illness and aging.
2000 years earlier to that, Hippocrates had written that “death resides in the bowels” and that “bad digestion is the root of all evil.”
Metchnikoff also proposed that toxins resulted from bacterial putrefaction in the colon, and then absorbed and released to the bloodstream are responsible for aging. He addressed these bacteria with the term “putrefying bacteria”, which is now known as proteolytic clostridia. Elie also stated that “the intestinal flora depend on the food taken by the host makes it possible to adopt measures to modify the flora in our bodies and to replace the harmful microbes by useful ones.” This statement clearly describes the “probiotic concept.”
Metchnikoff considered the lactobacilli as probiotics (“probios,” indicates “for life” of the host as opposed to antibiotics), which could have a positive impact on health and reduce aging. The scientific foundation of Metchnikoff was backed by the introduction and development of the first European dairy industry in France and innovating the use of a fermented milk attained from Bacillus bulgaricus.
In 2013, a paper was published on the scope and proper use of the term “probiotic” suggested that live microorganisms that are prescribed in which when administered in acceptable amounts can offer the host a good amount of health benefits.
Modern technology has also utilized those strains in the production of a fermented milk with good nutritional qualities, compared to other strains. According to a consensus by many science historians, yogurt and other food products made from fermented milk can be considered as the first functional foods.
History of probiotics is quite old throughout human history and is closely associated with food fermentations. Earlier in life, humans when had replaced hunting and gathering with farming, and from that, the critical need for food and beverages’ fermentation had been ensued. Sumerians were the first civilization to settle and develop animal breeding. In ancient Indian Ayurvedic texts, milk and dairy products consumption is associated with health wellness as well as longevity.
The earliest evidence on the practice appeared during the excavations of the city of Ur (Uruk), an ancient city in Mesopotamia and the birthplace of Abraham around 3100 BC. Almost every civilization had practiced food fermentation of some kind. Archeologists had found fermented beverage that dated back to 7000 BC in the Neolithic village of Jianhua in China and about 5000 BC in ancient Mesopotamia. In Asia, fermented beverages were mainly made from common foods such as rice, whereas in ancient Mesopotamia and Egypt it was made from fruits (e.g., wine), honey (mead), and from malted grains (beer).
The depictive and written scripts and evidence between 2000 and 3000 BC, even earlier than that, had shown that Egyptians, Hindu, Greeks, and Romans have used fermented milk products. They are mentioned in the early versions of the holy books of Hinduism and the Bible. In the Bible, one of the first statements is found in Genesis (18:1-8) where it is stated that Abraham offered the Lord “Veal, buns and sour milk.”
In ancient times there were struggle of humans to treat common disease, old Chinese doctors discovered that human or animal excreta, such as human or animal fecal matter, might be used as medicine. In traditional Chinese medicine had been found a written monograph illustrating: “Treatment of patients with food poisoning or severe diarrhea by ingesting human fecal matter suspension.
Nowadays fecal transplant has proven to cure Clostridium difficile infections even more efficiently than specific antibiotics, going far as preventing recurrence of such condition.
Gut microbiota
Essential points
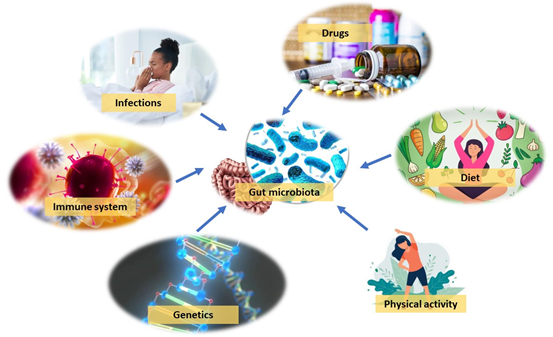
Figure 1 Factors influencing the gut microbial composition genetics, immune system, diet, drugs, physical activity, and infections. (Adapted and modified from Endocrine Review 2022, Vol.43, No.5).
Multiple studies have indicated the significance of diet for the microbial composition. One of the examples is to stop breastfeeding for infant when reaching a certain age, a requirement for the maturation of the infant microbiota towards an adult-like structure fortified in Bacteroides, Bilophila, Clostridium, Roseburia, and Anaerostipes . With adults, the diet is closely related to gut microbiome composition, and shifting from animal-based to plant-based diet can cause instant and significant changes in the gut microbiota. These changes depend on differences in the microbes’ capability in utilizing and metabolizing dietary components to serve their growth. On the other hand, even if short-term timeframe, radical changes in the diet can cause rapid changes in microbial composition. In addition, mild changes in diet can only result in slight changes in the gut microbiome. Other factors such as immune system, physical activity, infections, in addition to antibiotics and other medications, can also impact gut microbial composition. Moreover, host genetics have been shown to play a role as well. The gut microbiota composition was observed to be more similar among twins compared with unrelated individuals, and monozygotic twin pairs was observed to have more similar microbiotas compared to dizygotic twin pairs. Nevertheless, the impact of host genetics in shaping the gut microbiota is quite small, generally, environmental factors are more dominant factors yet explaining somewhat little variations in the microbiome.
The gut microbiota is not a group of independent microorganisms, instead it is a complex microbial ecosystem where these micro-organisms, cross-feed, communicate, co-develop and recombine. These largely unexplored, complex polymicrobial–host interactions are closely linked to the diet. According to the dietary composition in addition to presence of multiple microbes, series of metabolites are generated by these microbes. This indicates that even if two humans have the same diet, the metabolites produced can significantly vary according to their microbial composition. Individuals with similar gut microbiota but different diets could also possess different metabolites produced. These metabolites are vital for microbial interaction and cross-feeding; however, they could also have an impact on the host’s physiology via binding to related receptors in the host. Generally, metabolites mechanisms are still unknown from the aspect of how they are produced, the identical receptors, as well as the functions within the host. On the other hand, wide-scale metabolic screening techniques have lately been implemented to identify interactions among metabolites and receptors, thus improving our understanding and familiarity of these signaling pathways.
Colonization of “commensal microbes” and the interaction between these and the host are vital for the healthy development of the host. The benefits of the gut microbiota are development of immune tissues in addition to improving immune responses. Much of the metabolites and microbial components act locally and on different parts of the body thus regulating immune function as well as other physiological mechanisms. In addition to serving the immune system, commensal microbes bare an important role in preventing pathogens to colonize host by restricting space and nutrients for pathogens. Host–microbes’ symbiosis is also important for metabolic health. Microbes produce vitamins as human body lack the ability to perform biosynthetic function for most vitamins. Despite the availability of vitamins in food, deficiency can still occur attributable to malnutrition. In addition to the mentioned effects, the gut microbiota impacts metabolic functions in multiple organs such as the gut, adipose tissues (white and brown), liver, skeletal muscles, and the brain (Figure 2). These effects along with the therapeutic potential of addressing the microbiota will be included in detail in this review.

Figure 2 Metabolites produced by gut microbiota and their effects; direct on the gut and remote on other distant organs. (By permission from Endocrine Review 2022, Vol.43, No.5).
Clinical effects of probiotics
Mechanism of activity and efficacy
The gut microbiota offers essential capabilities for the fermentation of non-digestible substrates such as dietary fibers and endogenous intestinal mucus. This fermentation emphasizes the growth of specialized microbes that generate short chain fatty acids (SCFAs) as well as gases, butyrate, propionate, and acetate.
Butyrate is considered as the main energy source for colonic cells. Butyrate enhances apoptosis of colonic neoplastic cells, and can activate intestinal gluconeogenesis, which is beneficial for glucose and energy homeostasis.
In addition, Butyrate is vital for epithelial cells to utilize large portions of oxygen through β-oxidation, thus producing a state of hypoxia that can maintain oxygen balance in the gut, which will result in preventing imbalance in gut microbiota.
Propionate is transported to the liver, whereby it regulates gluconeogenesis and activates satiety signaling by acting on fatty acid receptors of the gut.
Acetate: is carried to the peripheral tissues where it can be utilized in cholesterol metabolism as well as lipid synthesis and thus play a role in regulating central appetite.
Multiple controlled trials have demonstrated that higher production of SCFAs corresponds to lower diet-encouraged obesity and with decreased insulin resistance. Only Butyrate and Propionate, not Acetate, shown to control gut hormones and thus reducing food intake and appetite and in mice.
Enzymes are produced by gut microbiota reduce bile acids generating unconjugated and secondary bile acids that ultimately play a role as signaling molecules and metabolic regulators to control vital host physiologic pathways.
Probiotics have different mechanisms of action though the actual ways of their biological impacts still yet to be understood.
These range from:
The last point has been the subject of many studies and there is substantial evidence that probiotics can influence multiple aspects of the acquired and innate immune response by: stimulating phagocytosis and IgA generation, altering T-cell responses, improving Th1 response, and reducing Th2 responses (Figure 3).
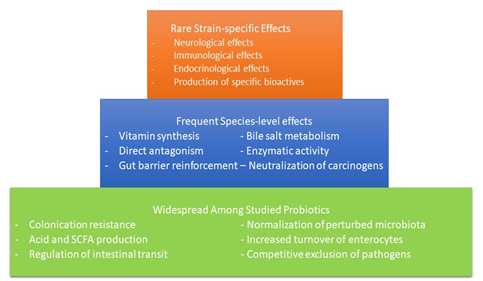
Figure 3 Possible distribution of mechanisms among probiotics. SCFA, short-chain fatty acid. (Permission: NATURE REVIEWS: Gastroenterology & Hepatology Vol.11 AUG. 2014).
General health benefits
There is growing evidence favoring the claims of positive effects credited to probiotics such as improving intestinal health, regulating the immune response, decreasing serum cholesterol, and neoplastic prevention (Figure 4).

Figure 4 Schematic representation of the role of the gut microbiota in health and disease giving some examples of inputs and outputs. CVD=cardiovascular disease; IPA=indole propionic acid; LPS=lipopolysaccharide; SCFA=short chain fatty acids; TMAO=trimethylamine N-oxide (adapted from Science and Politics in Nutrition, BMJ).
These health characteristics are strain-specific and are motivated by the various mechanisms. Some of the health benefits are well proven, while others require additional studies to be conducted. On the other hand, there is growing evidence to utilize probiotic in the treatment of acute diarrheal diseases, IBS and improvement in IBD, treatment and prevention of antibiotic-induced diarrhea ( C. difficile) , and improvement of lactose intolerance , urogenital infections in women and prevent recurrence.
Oral health
The mouth contains variety of microbes, bacteria, viruses, fungi and else. Bacteria trigger two popular diseases: dental caries and periodontal disease. The balance of theses microbiome can be disrupted and results in abundance of pathogenic microbiome that leads to different oral problems: dental caries, periodontitis, and halitosis.
For last two decades there is emergence of treating oral health problems by probiotics, like using certain species of lactobacillus and Bifidobacterium to reduce the levels of cariogenic species of streptococcus, and some can suppress microorganisms that cause pharyngitis or otitis media and tonsillitis. probiotics has been shown effective in treating distressing condition especially for women; halitosis and reducing the production of volatile sulfur compounds, which are the main culprit in this condition.
Probiotics and diarrhea
Antibiotic-induced diarrhea
Mild or acute episodes of diarrhea are some of the popular side effects of antibiotic treatment in which the normal microflora have a propensity to be suppressed, thus increasing the overgrowth of opportunistic or pathogenic forms. Spectrum may vary from diarrhea without mucosal abnormality to “pseudomembranous colitis”. The second one is a severe form of antibiotic-associated diarrhea (caused by toxin of Clostridium difficile, which may emerge after broad spectrum antibiotic use). This disorder is characterized by presence which plaques attached to mucosa that are fibrinous purulent exudates (pseudo membrane) and clinically it is considered by diarrhea, vomiting, abdominal distention, fever, and leukocytosis. In addition, if left untreated, this might result to serious complications, toxic dilatation, perforation in the colon and systemic upset.
Therapy comprises of withdrawal of offending antibiotic, supervision on the electrolyte imbalance, and in acute cases, implementing antimicrobial therapy including metronidazole or vancomycin is required. Probiotics treatment has been recently employed in clinical practice with L.rhamnosus and S. boulardii being administered. Multiple studies that have been conducted suggested that probiotic use in this case is linked with a decreased risk of antibiotic-related diarrhea.
Probiotics in immunity and infection
There is a plethora of evidence suggesting that the gut microbiota can regulate the intestinal immune system. Multiple probiotic strains have demonstrated to have the capability to target the immune system and, sometimes modulate intestinal immunity.
On the other hand, the clinical outcome of this situation is not firmly established. Some studies have demonstrated inconclusive evidence on the therapeutic effects of probiotics on some immunological and allergic conditions. Evidence is most likely difficult to reach here than in other clinical arena, for that reason, it is likely that the potential effect may vary according to the target, timing of probiotic intake, doses, and many others. In addition, there is evidence from preventive studies suggesting that some probiotics can shorten the course of some respiratory tract infections and thus reducing the difficulty of symptoms, however, they do not control the incidence of infections.
Probiotics and IBS (irritable bowel syndrome)
There is a floor of evidence for some probiotic strains that are associated with improvements in overall IBS symptoms, especially bloating and abdominal pain. The nature of the endpoints is subjective more than being objective, thus studies in this regard should be well controlled and double-blind, nevertheless the recent systematic review and metanalyses of randomized controlled trials have shown significant benefit (though modest) of the tested probiotic strains over placebo with significant heterogeneity. Updated therapeutic options with the capability to modify intestinal microbiota have recently been identified in addition to include the low fermentable, oligo-, di-, monosaccharides, and polyols (FODMAP) diet, antibiotics, and probiotics. Probiotics may affect intestinal barrier function and exert anti-inflammatory actions. Many of the recent clinical studies have investigated the effects of probiotics in IBS patients, majority of these studies shown that probiotics use is effective in IBS patients. Because of the differences in the study designs (size, period of treatment, probiotic doses, and strains employed), clinical studies addressing the effectiveness of probiotics in IBS are challenging to compare, yet there are robust outcomes in favor of overall beneficial of probiotics in IBS.
There should be significant analyses required to highlight optimal treatment regimens (effective probiotic strains, single or combination administration), in addition to identifying subgroups of patients presumably can benefit from such treatments.
Specifically, Probiotics are an effective treatment among IBS patients.it is considered good alternative option to the conventional treatment and worth’s trying, as the existing treatments carry wide variability of clinical results. Single probiotics at a low dose and short treatment duration appear to be more effective in improving overall symptom response and QoL.
Probiotics and IBD (Inflammatory Bowel Disease)
Recent reviewed and analyzed studies, have demonstrated that several probiotics strains can prolong remission in ulcerative colitis, these results would deserve further investigations to establish the type of strains, single or combo, dose, and duration. Some current trials have indicated additional effectiveness when co-administered with standard treatments, however, no promising results were obtained from probiotics implemented to Crohn’s disease patients.
Vaginal microbiome
World Health Organization (WHO) has demonstrated that Probiotics are live microorganisms that if managed in sufficient amounts, can offer substantial benefit to human health. Media tends to show probiotics as promising health method with the capability to prevent or treat a wide spectrum of health conditions. In gynecology, Lactobacilli species are basically used to repair the physiologic vaginal microbiota to treat bacterial vaginosis as well as vulvovaginal candidiasis and prevent premature birth. Over the last decade, much clinical research has been conducted to examine the effects of probiotics in prevention and treatment of a broad spectrum of health conditions, and the scientific attention in this field is increasing. Additionally, probiotics prescribed orally and vaginally have been implemented for preventing and treating vaginal infections as well as premature births. The concept of utilizing oral probiotics in the treatment of gynecological disorders is linked to the capability of these microorganisms to survive through the gastrointestinal tracts and to transport to the vaginal canal after their excretion from the lower gut. On the other hand, vaginal intake allows a targeted colonization action of the probiotics for restoring healthy vaginal microbiota. The vaginal microbiome is quite complicated and dynamically changing environment that consistently experiences fluctuations during the woman’s life and specifically during the menstrual cycle. The superficial layer of the vagina consists of stratified squamous epithelium which is coated by film of cervicovaginal secretions. The vaginal mucosa extracts their oxygen, glucose, and other nutrients from the underlying submucosal layer via diffusion, due to the limited blood supply in the surrounding area. As a result, this can create a relatively anaerobic medium.
Vaginal microbiome is defined as “the microbial community that live in a symbiosis (commensal) with the host”. In reproductive-aged women, physiological changes as hormonal changes, this can cause fluctuations in the composition of the microbiome. Lactobacillus species thrive in the vaginal anaerobic environment and generate various components, such as lactic acid, hydrogen peroxide and bacteriocins, therefore contributing to a healthy vaginal microbiome and constructing a defensive barrier against invading pathogens. Significant differences have been described among pregnant and non-pregnant women with regards to the vaginal microbiome. According to the results, a sharp decrease in the diversity and availability of the vaginal microbiome is seen in pregnant women. In addition, the prevalence of Lactobacillus spp., Actinomyceta, Clostridia, and Bacteroida is remarked in pregnant women. However, in non-pregnant women, the prevalence of Lactobacillus spp., Actinobacteria, Prevotella, Veillonella, Streptococcus, Proteobacteria, Bifidobacteria, Bacteroides, and Burkholderia is remarked, therefore suggesting that the vaginal microbiome would temporarily change in the same individual.
In addition to the vaginal microbiome differing largely among women, and the differences are credited to variations in sexual activity, chronic stress, cleansing, regional variations, ethnicity, and some other factors.
Research had been implemented among 396 concerned women (North American). They were healthy, asymptomatic, and coming from four ethnic groups, have shown that most vaginal microbiomes are dominated by single or even multiple Lactobacillus species and are categorized into five CSTs (community state type); CSTs I, II, III, and V are dominated by L. crispatus, L. gasseri, L. iners, and L. jensenii, respectively, however the CST IV refers to high variety of the microbial community described by strict anaerobic bacteria.
The differences in vaginal microbiome by women’s ethnicity might be associated with host genetic factors, for example, immune system, receptor’s ligands of epithelial surface, and the amount and structure of cervicovaginal discharge. When comparing behavioral and cultural differences, host factors might play a vital role in shaping the vaginal microbiome among different races.
Lactobacillus species thrive in the vaginal anaerobic environment and generate multiple compounds including lactic acid, hydrogen peroxide and bacteriocins, in which they contribute positively to a healthy vaginal microbiome and thus establishing a defense barrier towards harmful organisms. Lactobacillus species are the main supplier of L-lactic acid and D-lactic acid that can both regulate the pH level of the vaginal milieu to lower than 4.5, whereas epithelial cells provide just about 20% L-lactic acid.
Gardnerella is the most popular microorganism obtained from the women vagina with Bacterial Vaginosis. G. vaginalis was assumed that the only microbe of this species that can cause BV, while other strains have been considered responsible for this pathology as well.
Probiotics in gynecology
Non-pregnant women
Vaginal infections are considered one of the most popular reasons for gynecological consultation. It is anticipated that approximately 7 out of 10 women will encounter at least one episode of vulvovaginal candidiasis (VVC) during their lives. Bacterial vaginosis (BV) is another highly common vaginal disorder linked with an increased potential for pelvic inflammatory disease, STDs, HIV transmission, and premature births. Bacterial vaginosis is described by a reduction or depletion of lactobacilli and overgrowth of Gardnerella vaginalis, Mycoplasma hominis, Prevotella species, and other obligate anaerobic bacteria. Lactobacillus species generate both lactic and acetic acid as well as hydrogen peroxide in order to maintain the vaginal pH of around 4.5 or less, thus preventing growth of pathogenic bacteria and Candida albicans, so they are considered protective against VVC and BV. Therefore, the established beneficial effect of Lactobacillus species-containing probiotics in retrieving and maintaining healthy vaginal microbiota, affirming their use for the treatment of both vaginal disorders (Table 1).
|
Microorganisms considered as probiotics |
|
|
Lactobacillus species |
Bifidobacterium species |
|
L. acidophilus |
B. adolescentis |
|
L. casei |
B. animalis |
|
L. crispatus |
B. bifidum |
|
L. gallinarum1 |
B. infantis |
|
L. gasseri |
B. lactis2 |
|
L. johnsonii |
B. longum |
|
L. paracasei |
|
|
L. plantarum |
|
|
L. reuteri |
|
|
L. rhamnosus |
|
|
Other lactic acid bacteria |
Non-lactic acid bacteria |
|
Enterococcus faecalis1 |
Bacillus cereus var. toyoi1 |
|
E. faecium |
Escherichia coli strain nissle |
|
Lactococcus lactis3 |
Propionibacterium freudenreichii |
|
Leuconostoc mesenteroides |
Saccharomyces cerevisiae |
|
Pediococcus acidilactici3 |
S. boulardii |
|
Sporolactobacillus inulinus1 |
|
|
Streptocosccus thermophilus3 |
|
Microbial species applied as probiotics
The most acceptable beneficial effects of probiotics in treating BV have been demonstrated in many metanalyses and shown encouraging results (though inconsistent!) whether with oral or vaginal supplements combined with specific anti-microbial or without.
Pregnant women
It has been indicated that probiotics could play a significant role in preventing premature birth. Premature birth rates differ across different countries, from 5 to 9% in Europe, and 13% in Northern America. The etiology of premature birth is multifactorial, but it has been assessed that about a third of these cases is mainly due to intrauterine inflammation triggered by rising vaginal infections. A pre-existing BV tends to be highly associated with premature births. For that reason, the beneficial role of probiotics might be linked with their potential ability to displace (abundance effect) and feed competition, produce anti-inflammatory cytokines and even reducing vaginal pH changes and eventually will retrieve healthy milieu and eliminate invading pathogens.
In addition, the employment of probiotics in pregnancy could enhance maternal glucose metabolism via the variation of gut microbial composition and function, and the enhancement of insulin sensitivity.
The overall studies that had evaluated the effects of probiotics during pregnancy haven’t showed significant protection for most of secondary endpoints; gestational diabetes, premature membranes’ rupture and small or large infant’s gestational age, but clearly supported the positive glucose homeostasis and improved insulin sensitivity.
The current concept is directed toward increasing the population of healthy vaginal bacteria at the cost of potentially harmful microbes this concept is considered the main goal of the expanding marketing of probiotics around the world.
The question that is still unanswered and rendered debatable whether change in vaginal microbiota the result of incidental infection or systemic alteration in immunity, hormonal, or metabolic factors is that singly or collectively predispose to alteration in microbiome milieu.
Therefore, incorporating probiotics might restore the healthy microbiota and would result in curing the consequences rather than root of the problem. The considerable relapse rate after antimicrobial coupled with probiotics use of BV and VVC might suggest the second view more. Moreover, the route of remedies like vaginal route would result in more favorable outcome rather than oral route (Table 2).
Guideline |
Role of probiotics |
European (IUSTI/WHO) guideline, |
Potential role of vaginal probiotics in the management |
Faculty of Sexual & Reproductive |
Recurrent BV: |
There is currently insufficient evidence to recommend |
|
Recurrent VVC: |
|
Non-conventional management regimens such as dietary |
|
German Society for Gynecology |
Probiotics have shown encouraging, but controversial |
Society of Obstetrician and Gynecologist |
Current evidence of the efficacy of alternative therapies |
Centers for Disease Control and |
Overall, no studies support the addition of any available |
Table 2 Role of probiotics in international guidelines for the treatment of vulvovaginal infections
IUSTI/WHO = International Union against Sexually Transmitted Infection and the World Health Organization
Probiotics use rationale
The extensive use of probiotics to reduce the risk of premature birth and to increase the cure rate of BV and VVC does not seem to be explained by the current available data.
In fact, despite the increase in marketing and sales of probiotics, the results obtained by clinical trials are unreliable and generally suboptimal. In addition, a considerable number of these trials have been endorsed by parties with a commercial interest in the outcome (commercial bias). There was a considerable discrepancy in published studies from the aspect of strains of probiotics adopted, ways of administration (oral, vaginal), and period of treatment.
The effects of probiotics seem to be strain-specific and dose-dependent, and the lack of a standardized manufacturing mechanism could impact microbial being, growth, and sustainability.
Moreover, a recent position research paper done by The European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) Working Group provides proof on the low quality of commercial probiotic products from the aspects of definition of microorganisms, their numbers, functional properties, as well as presence of contaminating microorganisms.
The working group has suggested the following:
Therapeutic potential
There are accumulating evidences showing a fundamental role for gut microbiota and their products and metabolites in metabolic illnesses; Metabolic syndrome (Insulin Resistance), Obesity, T2 diabetes and liver steatosis, this potential open the subject for targeting gut microbiota towards those disorders. There are multiple ways which the gut microbial structure and function can be altered by dietary interventions and individuals react differently to interventions directed to the gut microbiota. Individuals with T2 Diabetes that already have high alpha diversity (diversity of species in a single ecosystem) comprising of a high number of butyrate producers may not serve benefit from specific probiotics as individuals with low alpha diversity and number of butyrate producers, as a result, the gut microbiota may permit patients’ classification and then appropriate individualized management.
Obesity is increasing health problem around the world with associated comorbidities; T2 diabetes, atherosclerotic cardiovascular diseases, and hepatic steatosis.
Multiple studies have investigated fecal microbial structure and the field of alteration of fecal microbes in obese people by transplant is widely opened the doors for research and therapeutic potentials. Another field of studies is that microbiome composition is closely related to many cardiometabolic markers, fasting and post-meal glycemic, lipemic and inflammatory parameters.
The gut microbiota utilizes local metabolic effects within the gut, where it impacts the ability to digest, extracting energy and nutrients, and excreting byproducts from ingested food. The functions are regulated by the microbiota include intestinal permeability and motility, and generation of gut hormones. On the other hand, the gut microbiota does not only have local impacts in the intestine (Figure 3). The microbes generate multiple small molecules that can impact the host’s physiology, both in the intestine and in other organs, adipose tissues, liver, skeletal muscles, immune system, brain.
International consensus
Consensus panel FAO/WHO recommendations for the scope of probiotics
Consensus panel recommendations for the scope of probiotics correction as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”.
For the past two decades, there has been build up in field of probiotics research, we have met with wide ranges of studies including, gut health, immune support, cholesterol and lipid control, energy and appetite control and may be sensorimotor behavior. there are tremendous works on animal models on the mechanisms of the action of probiotics, some have reached the results that gut microbiota can normalize inflammation, satiety, obesity, glucose metabolism and energy. We are anticipating that these results would be reflected to human health.
Presently, there are well structured studies that have proved the potential engagement of gut microbiota for preventing and treating serious diseases such as hypercholesterolemia and adiposity. Using fermented dairy products such as yoghurts and encapsulated bile salt like hydrolase were proven to be successful in animal trials. Recent marketed probiotic L. reuteri NCIMB for cholesterol lowering in human. Animals studied have shown by utilizing bioactive components of some microbiota associated with suppressed carcinogenesis at different sites. Studies that some microbiota can control serotonin release thus can modulate behavior and could be use in stress-induced CNS disorders.
Low fiber intake in diet reduces production of small chain fatty acids and shifts the gut microbiota metabolism utilizing unfavorable nutrients, leading to the production of potentially harmful metabolites, that eventually will be reflected on unhealthy response toward human physiology. Recent studies have shown that the detrimental effects of high fat diets that mimic western diet on integrity of the epithelial layer of the intestine and disturb the penetration.
In conclusion, based on the mentioned findings, along with the role of butyrate in preventing oxygen-induced gut microbiota dysbiosis, has offered a strong basis for improving dietary fibers consumption to sustain complete mucosal barrier. Fiber is a vital nutrient for a healthy microbiome and has been overlooked while issues of sugar and fat was taken more credit than needed.
There is close relation between a high diversity of vaginal microbiota and health of urogenital tracts. Probiotics have demonstrated a vital role in maintaining the health of the genital tract in females, improving common gynecological disorders, and the local immunity of the vagina. The utilization of probiotics or VMT intervention possesses a particular impact in preventing the development of cervical neoplasia, treating Bacterial Vaginosis, and reducing symptoms of atrophic (senile) vaginitis.
We require clinical evidence that can be implemented into clinical practice, preferably via randomized regulated studies that utilize available preparations of prebiotics, probiotics, or fecal microbiota transplantation to encourage changes in gut microbiota composition and therefore providing positive health outcomes.
Fecal microbiota transplantation (FMT) has gained popularity and attraction in the treatment of other diseases such as digestive system diseases and has also accomplished substantial results. In addition to that, recently, there has also been an increasing interest in vaginal microbial transplantation (VMT).
One study has investigated the impact of VMT on vaginal-disturbed microbiota by creating a vaginal dysbiosis model. The results demonstrated that VMT significantly decreased bacterial-induced inflammation, reduced inflammatory cytokines, and reestablished normal healthy vaginal microbiota.
Mr. Ammar S. AlJadir, BSc Communication Engineering & Computer Science, Siemens Energy, Baghdad, IRAQ.
None.
Author declares that there are no conflicts of interest.

©2023 AlJadir. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.