eISSN: 2377-4304


Objective: To report the prevalence of pNK≥12% and its relationship with RPL in a population of Mexican women.
Methods: Retrospective, cross sectional and observational study which included 98 women with history of RPL, who were subjected to a blood test sample to measure pNK cell and prevent complications in further pregnancies. Two groups were formed: 1) CONTROL and 2) RPL: Women with history of 2 or more miscarriages.
Results: Women in the RPL group were older and had higher BMI compared to the Control group. Prevalence of patient whit pNK≥12% increase in the RPL vs Control. RPL group showed a significant increase of pNK≥1% compared to the Control (11.3±0.5 vs 9.5±0.6%, p=0.002). Finally, the median level of pNK≥12% in the PRL group was higher than Control (15.1±0.5 vs 13.5±0.8%).
Conclusion: RPL may be the result of increased pNK concentrations and as observed in this study, slightly more than 50% of the Mexican population could be susceptible to abortions.
Keywords: recurrent pregnancy loss, natural killers, spontaneous abortion, missed miscarriage, anembryonic, chemical pregnancy
Recurrent Pregnancy Loss (RPL) is a diagnosis of concern for many physicians in the fertility field. Currently, the American Society for Reproductive Medicine (ASRM) defines RPL as the loss of two or more clinical pregnancies up to 20 weeks of gestation; and by the European Society of Human Reproduction and Embryology (ESHRE) as two or more miscarriages up to 24 weeks of gestation (including chemical pregnancy).1,2 It affects about 0.5 to 2% of couples,3 and is associated with anatomical,4 autoimmune,5 endocrine,5 genetic,6 infectious,6 haemathological (thrombophilia7), and masculine factor,8 being 45-50% cases of unidentified etiology (idiopathic factor).9
Some immunological conditions such as unbalance of Th1 and Th2, autoimmunity, increase of Natural Killer (NK) cells, as well as some combinations of KIR and HLA embryonic receptors, which disturb the immune balance during pregnancy, have been associated with RPL.9
NK cells are lymphocytes belonging to the innate immune system with heterogeneous populations. Some of their main functions are cytotoxicity and cytokine production. Regarding reproductive level, two of the most important populations are: 1) peripheral blood NK (pNK) cells, which contain protein granules involved in lytic activity, and a small amount of cytokines. Their increase in number as well as in activity has been associated to RPL since their main function is the defense against tumoral cells by means of cytotoxicity and apoptosis;9,10 and 2) uterine NK (uNK) cells, which have a low cytotoxic ability and produce high concentration of cytokines, chemokines, and growth factors involved in regulating the immune microenvironment which is related to the control of trophoblast invasion, the angiogenesis process, and remodeling of spiral and uteroplacental arteries.9,11–14 Altogether, pNK cells level increase may cause reproductive complications such as RPL and RIF.15,16
Some of the treatments used to regulate NK levels include intravenous gamma-globulins (IVGG), corticoids, anti-TNF drugs, Intralipid I.V. infusion, GMCS-F (Granulocyte-Monocyte Colony-Stimulating Factor), PF (preimplantation Factor), Lymphocyte I.V. therapy, immunotherapy, and Vitamin D supplementation [9]. Particularly, evidence from therapy with parental lymphocytes I.V. has shown to promote maternal immunomodulation by regulating the levels of pNK, and uNK cells.17 This assists embryonic implantation and avoids pregnancy loss.18,19
Due to the foregoing, this work aims to report the prevalence of pNK≥12% in patients with history of RPL, describing its relationship with literature reports.
Retrospective cross sectional and observational study which included 98 women with history of RPL seen in PRONATAL clinic (Bité Medical Hospital, Mexico City), who were subjected to measurement of pNK cell levels in order to prevent complications in further pregnancies.
Anthropometric data (age, weight, height, and BMI) as well as obstetrical history, were taken from the medical records and collected by the nursery staff on the patient’s first visit. Levels of pNK cells were taken from peripheral blood sample in the middle of the luteal phase (day 19-23). Samples were sent to Diagnomol laboratory, where the pNK cell count (CD16+ CD56+) was made by flow cytometry.
With the gathered data two groups were formed: 1) Control (n=20): women with at least 1 spontaneous full-term pregnancy with no history of complications, pregnancy loss or implantation failure; 2) RPL (n=78): women with history of 2 or more pregnancy losses [Spontaneous Abortion (SA), Missed Miscarriage (MM), Anembryonic or Chemical], with or without background of one or more pregnancies (preterm and full-term).
Inclusion criteria: Women in reproductive age with evaluation of pNK-cell (CD16+ CD56+) level, complete record [age, weight, size, BMI, and pNK-cell (CD16+ CD56+) levels], idiopathic RPL.
Exclusion criteria: Patients with no pNK-cell (CD16+ CD56+) study, women who did not consent to participate in the study, women with RPL associated to anatomical, endocrine, infectious, genetic, masculine factors, and thrombophilia, as well as women with systemic, genetic, neoplastic, thyroid diseases, diabetes mellitus, polycystic ovary syndrome. Variables to be studied: pNK-cell levels and RPL.
All patients were informed about the use and managing of the collected data (age, weight, size, personal data, and pNK cell results) which allowed their inclusion in this study. In addition, their anonymity was respected because there was no tracing related to the origin of the data, and only numerical and statistical data (as the case might be) were published.
Levels of pNK-cell, weight, and size of the patient were reported as the mean () ± standard error (SE), and were subjected to Student’s t test. On the other hand, vaginal delivery, caesarean section, pregnancy losses (SA, MM, Anembryonic, and Chemical), and pNK cell prevalence were reported as percentages (%) and were submitted to Chi-squared test. In both of the cases, the statistical software SPSS version 25 was used.
Anthropometrical data of 98 women divided in a Control (n=20), and RPL group (n =78), were analyzed. The RPL showed a statistically significant increase in age compared to the Control (35.6±0.3 vs 35.1±0.9, p=0.00). Patients’ weights in the RPL group turned to be numerically higher than those in the Control (57.9±0.6 vs 53.6±1.2). Heights of RPL were statistically shorter compared to the Control (1.60±0.004 vs 1.61±0.01, p=0.00). As a result of these data, the BMI was statistically higher in the RPL compared to the Control (22.4±0.2 vs 20.4±0.1, p=0.00) (Table 1).
|
|
Control |
RPL |
p value |
|
n |
20 |
78 |
- |
|
Age (years) |
35.1±0.9 |
35.6±0.3* |
0 |
|
Weight (kg) |
53.6±1.2 |
57.9±0.6 |
0.55 |
|
Height (m) |
1.61±0.01 |
1.60±0.004* |
0 |
|
BMI (kg/m2) |
20.4±0.1 |
22.4±0.2* |
0 |
Table 1 Anthropometric measurements of mothers
*Statistically significant difference of RPL vs Control determined by Student’s t test, with p≤0.05
In the obstetrical background, RPL was associated with a lower prevalence of vaginal delivery (4.4 vs 69.4%, p=0.00), and caesarean delivery (11% vs 30.5%, p=0.00) compared to the Control. Caesarean deliveries were higher than vaginal deliveries in the RPL (11% vs 4.4%). In addition, as the methodology indicated, only RPL showed abortions, being the SA the most prevalent (44.1%), followed by MM (30.5%), Anembryonic (5.8%), and Chemical (4%) (Graph 1).
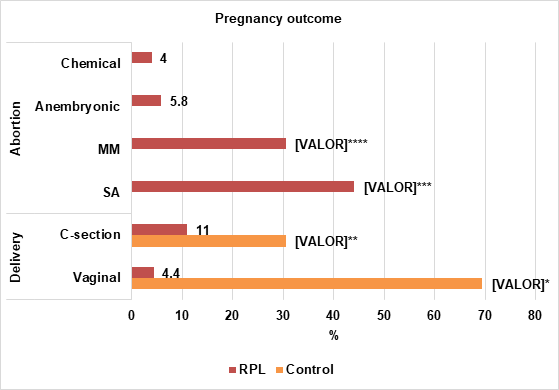
Graph 1 Prevalence of type of delivery (vaginal and C-section) in RPL and Control groups. Abortion rates (SA, MM, anembryonic and chemical abortion) are also shown. *Significant difference of vaginal delivery (RPL vs Control), **significant difference of Caesarean delivery (RPL vs Control), ***significant difference of SA vs MM, Anembryonic, and Chemical, ****significant difference of MM vs SA, Anembryonic, and Chemical abortion. Chi-squared test, p≤0.05.
Prevalence of patients with pNK≥12% showed increase in the RPL compared to the Control (56.4 vs 20%) (Graph 2).

Graph 2 Prevalence of women that showed pNK cell levels equal to or higher than 12% in the Control and RPL groups.
Finally, when the evaluation of ±SE of pNK≥1% cell levels from all patients was made, it was found that the RPL showed a significative increase of pNK cells compared to the Control (11.3±0.5 vs 9.5±0.6%, p=0.002). Furthermore, when only women with a level of pNK≥12% were evaluated, the RPL showed a higher level of pNK than the Control (15.1±0.5 vs 13.5±0.8%). In contrast, when only women with a level of pNK<12% were evaluated, the RPL showed a lower values compared to the Control (6.4±0.4 vs 8.5±0.4%) (Graph 3).
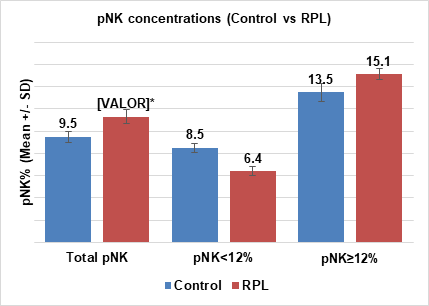
Graph 3 This graph shows Total pNK cell levels (±SE) (all patients), pNK<12% (only patients with NK cell levels lower than 12%), and pNK≥12% (only patients with NK cell levels equal to or higher than 12%); *Statistically significant difference of the RPL compared to the Control; Student’s t test, p≤0.05.
Currently, the mechanisms by which the immune system is regulated during pregnancy are not completely dilucidated. It has been described that embryos implanted in uterus show antigens (HLA-G) that, when identified by the immune system, trigger a signalization cascade that gives priority to cytotoxins and Th2, Th3, and Th1 lymphocytes. This generates an immune privilege for the embryo, which prevents the mother’s immune system to attack self antigens and those of the father.20 The immune privilege acquired by the embryo is sometimes lost because of immunological disturbances that result in gestational loss, just as it has been observed in mice with NK and regulatory T (Treg) cell depletion, which could not bred, and showed abortions continuously.21–23
NK cells are originated from bone marrow stem cells which, when differentiated, reach the blood stream, where it is thought that by interacting with certain molecules (integrins and adhesion molecules) induce migration of pNK cells to the endometrial and decidual stroma, and differentiate to uNK cells.24 Although most pNK cell immunophenotypes are different, endometrial NK cells have shown a deep relationship between these two populations, exposed in some papers as predictors of the state of uNK cells, and as a possible predictor of RPL.24–28
In this study, we found that women with RPL showed a higher level of pNK cells in the luteal phase of the menstrual cycle, when patients with pNK≥1 are compared with the Control (11.3±0.5 vs 9.5±0.6%, p≤0.05). In addition, when only women with pNK≥12% are included, an increase in NK levels is still observed in the RPL compared to the Control (56.4 vs 20%) (Graph 2) (Graph 3). In literature it has been found that up to 37.3% of patients with RPL show a small to moderate increase of NK cells, and up to 14% of women with RPL have shown a higher increase of pNK (56+) cells.29–31 Similarly, Toth B. (2019) who included 575 patients in his study, observed a higher percentage of pNK cells at the middle of the luteal phase in 393 women with RPL and primary infertility, compared with 182 patients with RPL and secondary infertility (12.4±5.5 vs 11.1±4.6, p=0.001).32 Sung K. (2019) found that 93.7% of women with history of RPL showed levels of pNK cells above 18% compared to the Control, where no patient showed a level of pNK cells higher than 18%.33 Adib Rad H. (2018), evaluated levels of pNK cells (9.25 vs 6.11) and uNK cells (6.80 vs 4.41) at the luteal phase and found that said levels were higher in patients with RPL than in the Control.34 All of this may lead to think that indeed the increase of pNK cells is directly related to their own reactivity and the activity of uNK cells.
Unfortunately, the studies carried out so far have shown very heterogeneous results, with findings where no apparent difference in pNK cell levels among patients with RPL and Controls exists, as shown by Yong Z. (2020) in patients with RPL, where no significative difference of pNK cell levels was found in samples evaluated at the middle of the luteal phase, compared with the Control (13.4±7.6 vs 14.2±7.1%).35 Concordantly, Strobel L. (2021), did not find a significant difference in patients with RPL when levels of pNK cells were evaluated in comparison to the Control (16.7±6.9 vs 15.9±6.3%).36 Azargoon A. (2019), reported no difference of pNK cell levels in the middle of the luteal phase in patients with RPL compared to the Control (15.9±5.1 vs 13.2±5).37 In addition, this heterogeneity not necessarily indicates that the increase of number or level of pNK cells will be accompanied by a higher cytotoxic activity that might affect pregnancy.38,39 Although our study reflects only data from a small population attending to Pronatal clinic in Mexico City, we were able to observe that an increase in pNK cells ≥12% might be associated to RPL. This makes us think that, in the afore-mentioned studies where the authors did not find a significant difference of pNK cell levels between RPL and Controls, the latter, despite of not showing disturbances in previous pregnancies, showed pNK cell levels ≥12%, might be susceptible groups since they showed pNK cell levels similar to those of RPL patients.
Finally, as it has been observed, there are just a few studies that evaluate the relationship between pNK cells and RPL, and have not reached a consensus that reflects the protagonist roll of NK cells in RPL; hence, the importance of reporting the results obtained in PRONATAL clinic. Even so, it is necessary to carry out more prospective studies with larger populations which would allow improving methodology, leading us to verify whether the pNK cell level ≥12% is indicative of RPL.
A little more than 50% of Mexican women with a history of RPL might have pNK cell levels ≥12%, which may be indicative of higher risk of pregnancy loss if all of these women would react similarly to those attending to PRONATAL clinic in Mexico City. Data reported in this study reflect a small population, and for this reason it is necessary to carry out more studies in Mexican populations to verify what was found in this work.
None.
None.
The authors report no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.