eISSN: 2377-4304


Review Article Volume 11 Issue 2
1Director and Consultant, Shri Lakshmi Clinic and Scan Centre, Department of Fetal Medicine, India
2Consultant, Shri Lakshmi Clinic and Scan Centre, Department of Fetal Medicine, India
Correspondence: Dr. Selvaraj Ravi Lakshmy M.B.B.S, Director and Consultant, Shri Lakshmi Clinic and Scan Centre, Department of Fetal Medicine, 1/350A, Amman nagar, Chennai Bye-pass, Krishnagiri - 635001 Tamil Nadu, India, Tel 9842850772
Received: January 23, 2020 | Published: April 1, 2020
Citation: Lakshmy SR, Thasleem Z, Parthasarathy P, et al. Low lying gestation sac in early pregnancy-an algorithmic approach with ultrasound markers. Obstet Gynecol Int J. 2020;11(2):107-114. DOI: 10.15406/ogij.2020.11.00496
The differential diagnosis of low lying gestational sac in ultrasound at early pregnancy scan varies from benign entities like cervical stage of miscarriage to morbid conditions like caesarean scar pregnancy and cervical pregnancy. Abnormal placental invasive pregnancies also manifest as low lying gestational sac at cervico-isthmic junction. Early diagnosis using simple ultrasound criteria can prevent severe life-threatening complications.
Various clues on ultrasonography can easily differentiate between a caesarean scar pregnancy, cervical pregnancy, cervical stage of miscarriage or a low implanted sac which may be a precursor for morbidly adherent placenta. In this article we have reviewed the ultrasound markers in a case of inevitable abortion, a case of cervical pregnancy, 3 cases of caesarean scar pregnancy and 2 cases of morbidly adherent placenta. Comparison of anterior myometrial thickness in a low implantation of sac and caesarean scar pregnancy has been demonstrated. Sometimes a small cervical polyp can mimic a low lying gestation sac and the differential diagnosis with ultrasound has been discussed.
Three dimensional ultrasound examinations for spatial location of the sac provide additional information for further management and aids in precise diagnosis. Though other investigations like MRI may also be beneficial in arriving at a diagnosis, its routine usage may be limited due to the cost and availability. Hence defining ultrasound markers in a low lying gestational sac helps in early diagnosis which can prevent fertility losses incurred due to inadvertent management
Keywords: caesarean scar pregnancy, cervical pregnancy, cervico-isthmic implantation, morbidly adherent placenta, first trimester, early pregnancy
CSP, caesarean scar pregnancy; CP, cervical pregnancy; MAP, morbidly adherent placenta
A low lying gestation sac on routine early pregnancy ultrasound may be the only finding in a spectrum of benign to morbid conditions. Abnormal implantation of the gestational sac in the lower uterine segment can be a life threatening condition like cervical pregnancy (CP), or caesarean scar pregnancy (CSP) or a cervico isthmic implantation leading to subsequent formation of morbidly adherent placenta. Cervical stage of miscarriage and cervical polyp which are benign conditions can also mimic these entities. Vaginal bleeding during the first trimester of pregnancy is the commonest presenting symptom in all these entities. The lack of awareness about the differential diagnosis of these conditions leads to misdiagnosis and inappropriate interventions leading to high maternal mortality and morbidity. The need to differentiate these entities is highly mandatory for optimizing antenatal care especially in conditions like cervical pregnancy, caesarean scar pregnancy and morbidly adherent placenta (MAP). Hence, the usage of definitive ultrasound criteria is the mainstay in precisely diagnosing these entities.
The criteria for cervical pregnancy would be the demonstration of the gestational sac or placenta predominantly in the cervix and hourglass shape of uterus with ballooned cervical canal. For a CSP, the ultrasound criteria required for diagnosis includes an empty uterus and an empty cervical canal with sac located in the anterior part of the uterine isthmus surrounded by insufficient myometrium. A prominent peritrophoblastic flow demonstrated around the sac on color Doppler suggests functional trophoblastic circulation and helps to distinguish from a non-viable detached intrauterine pregnancy. The “sliding sign” on transvaginal ultrasound examination serves as an additional finding to differentiate inevitable abortion from the other types. The differential diagnosis of low lying gestation sac is based almost solely on ultrasound findings rather than history and clinical examination. Hence an algorithmic approach to the differential diagnosis of low lying gestation sac based on ultrasound markers is discussed in this review.
An abortion in progress or the inevitable abortion is the condition where the products of conception have detached from the implantation site and continuation of pregnancy is not possible. The ballooning of the cervical canal due to retention of the abortus by the resistant external os in spontaneous abortion can rise the suspicion of cervical pregnancy.1 A non-viable sac passing through the cervix will have no peritrophoblastic flow and this serves as an initial clue. Figure 1 is an illustration of cervical stage of miscarriage, showing an open external os with products of conception in the cervical canal with absent peritrophoblastic flow. Short-term follow-up of a failed pregnancy will demonstrate lack of interval growth, and confirm that the gestational sac is not fixed in location and will have a positive sliding sign.2 Both cervical ectopic pregnancy and CSP often contain live embryos with detectable cardiac activity, while an abortion in progress will not. A combination of gray scale and colour doppler findings helps to differentiate the two entities.3,4
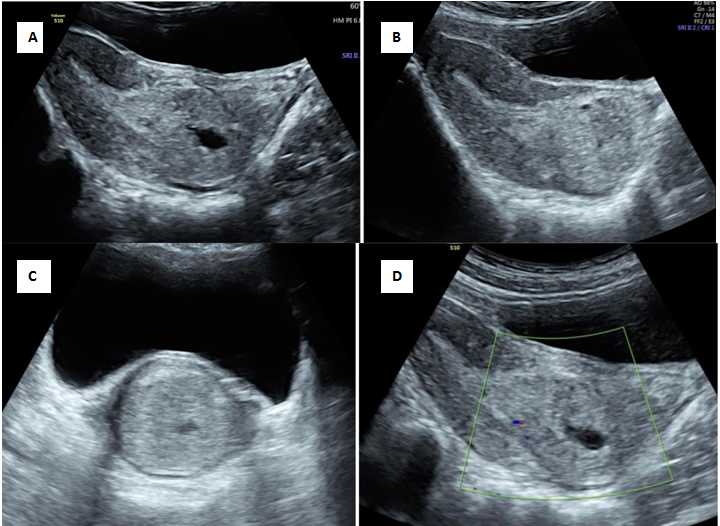
Figure 1 Cervical stage of miscarriage.
A) Transabdominal ultrasound showing echogenic mass seen in the cervical canal with splaying of anterior and posterior lip of cervix[*] B) Demonstration of open external os with products of conception in cervical canal C) Transverse section of cervix showing distended cervix with echogenic mass D) No evidence of peritrophoblastic vascularity seen surrounding mass.
The incidence of cervical pregnancy (CP) is less than 1% of all ectopics with the incidence varying from 1:1000 to 1:18000 pregnancies.5–8 Though cervical pregnancy was first described and named in the 18th century, the anatomical and histological criteria for the diagnosis of cervical pregnancy was defined later in 1911.9,10 On a hysterectomy specimen, the attachment of trophoblast must be below the level of entrance of uterine vessels to the uterus or anterior peritoneal reflection and products of conception must be absent from the corpus uteri.
Patients with cervical pregnancy generally present with painless first trimester vaginal bleeding, though some cases have cramping pain and are often misdiagnosed as abortion.9,11 The etiology of cervical pregnancy is unknown, and is proposed likely to be a result of a combination of factors, including local cervical pathology mainly of iatrogenic origin such as previous dilatation and curettage, Asherman‟s Syndrome, previous cervical or uterine surgery and in vitro fertilization or embryo transfer.12,13
Figure 2 is an illustration of cervical pregnancy .On ultrasound the gestational sac is present in the cervix (Figure 2A), an intact part of cervical canal exists between the sac and the closed internal os (Figure 2B) with peritrophoblastic flow (Figure 2C).14,15 3D ultrasound depicts the typical hour glass appearance of cervix. (Figure 2D) A mere presence of sac in the cervix could also indicate the cervical stage of abortion, and can be differentiated by demonstration of sliding sign on ultrasound.2
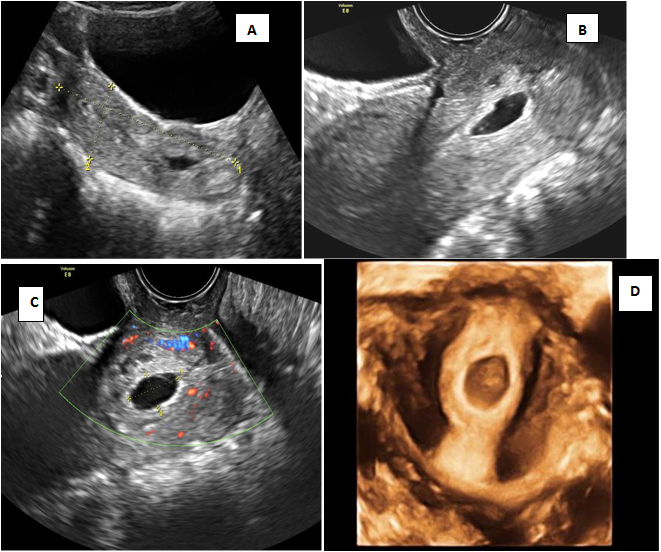
Figure 2 Cervical pregnancy (CP)
A) Transabdominal ultrasound shows the gestational sac within the cervix and empty uterine cavity B) Transvaginal ultrasound shows the gestational sac within the cervix with an intact part of cervical canal existing between the sac and the closed internal os (arrow) C) Peritrophoblastic flow surrounding the sac. D) 3D ultrasound showing hour glass appearance with closed internal and external os.
The important criteria for cervical pregnancy are the visualization of a gestational sac or trophoblastic mass below the level of the internal cervical os. In some cases the internal os is not clearly visualized due to increased endometrial thickness and echogenicity. In such cases a sac seen below the level of the uterine artery insertion itself can serve as a clue in finding cervical pregnancy as the uterine arteries insert at the level of internal os.2 If the gestation contains no sac or no living embryo, it is difficult to make a definitive diagnosis on a single U/S examination and needs a repeat evaluation. Sometimes, a small cervical polyp may mimic a sac and a case (Figure 3) referred as cervical pregnancy with a typical sac like structure in the cervical canal was actually a cervical polyp. Absence of the echogenic trophoblastic tissue surrounding the sac and vascularity in the pedicle of the polyp may help in the diagnosis. Early CP with its small ectopic size rarely presents with a typical hourglass shaped uterus. Besides, it is worth mentioning that the implantation site may occur in the high, middle, or low portion of the entire cervix, resulting in a varied location of sac along the cervical canal.15 Although fetoplacental remnants can be identified early in gestation with application of MRI, this tool remains inconvenient and limited due to its availability and high cost.16 Important diagnostic clue for a cervical pregnancy is that there would be a layer of healthy myometrium visible between the bladder and the gestation sac unlike caesarean scar pregnancy.17 Peritrophoblastic flow in CP which can be assessed using color flow Doppler is useful in both diagnosis and monitoring of treatment in cervical pregnancy.1,18–21
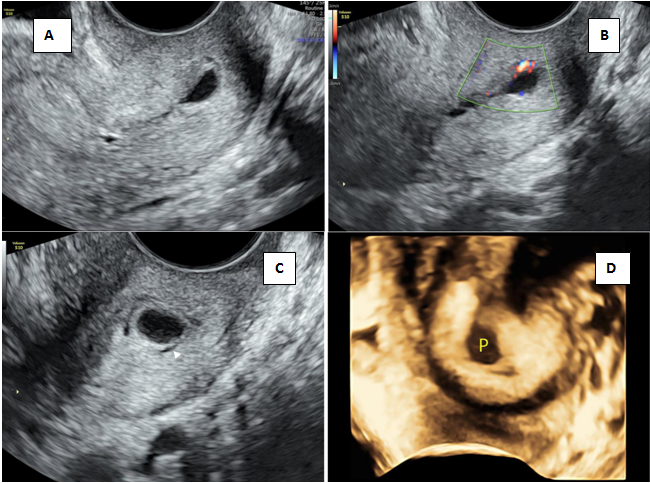
Figure 3 Cervical polyp mimicking low lying sac.
A) Transvaginal section of cervix showing sac like structure B) Eccentric vascularity seen in pedicle of polyp C) Polyp seen in the cervical canal with thin rim of fluid (arrowhead) D) 3D rendered image of polyp.
Sudden onset of massive bleeding from the implantation site likely occurs when detachment of the CP from the cervix occurs due to surgical manipulation and causes tearing and breaking of the underlying vessels that mainly originate from the uterine artery. The cervix is naturally less capable of fibromuscular contraction, which makes it more difficult to control bleeding and may sometimes need uterine artery ligation as an emergency rescue procedure.22 Hence mistaking a cervical pregnancy as cervical stage of miscarriage might lead to life threatening complications.
In CSP, the gestational sac is fully or partially implanted within the scar caused by a previous caesarean section.23,24 It has been estimated that 6.1% of pregnancies in women with at least one previous caesarean section (CS) and a diagnosis of ectopic pregnancy will be CSP.25 The increasing incidence of CSP is attributable partly to the increasing number of CS performed and also to increasing awareness and better ultrasound diagnosis. Literature reveals that up to 13.6% of CSP may be misdiagnosed as either inevitable miscarriages with a lowlying sac or cervical pregnancies.21,26 The most probable mechanism explaining scar implantation is invasion by the implanting blastocyst through a microscopic tract that develops from the trauma of an earlier CS.27,28
CSP can be classified into two types based on imaging findings and pregnancy progression.29,30 Type 1 or endogenic CSP is where implantation occurs on the scar and the gestational sac grows towards the uterine cavity. Type 2 or exogenic CSP occurs when the gestational sac is deeply embedded in the scar and the surrounding myometrium and grows towards the bladder having a greater risk of earlier rupture. A very thin myometrium between the bladder wall and the gestational sac is one of the most important prognostic predictor in CSP.31
Ultrasound criteria for diagnosis of caesarean scar pregnancy (CSP) are an empty uterine cavity and empty cervical canal, placenta or a gestational sac embedded in the scar of a previous caesarean section and a thin or absent myometrial layer [1 to 3mm] between the gestational sac and the bladder.32 Evidence of functional trophoblastic tissue on colour flow doppler examination is characterised by high velocity and low impedance peritrophoblastic blood flow.
Figure 4 is an illustration of caesarean scar pregnancy with echogenic trophoblastic tissue implanted in the scar site with anterior myometrial thinning and peritrophoblastic flow. Figure 5 is 3D illustration showing ballooning of the cervico-isthmic region and normal appearing cervix (compare with Figure 2D of CP). Multiplanar imaging showing deficient anterior myometrium at the uterovesical interface (Figure 5C & 5d).
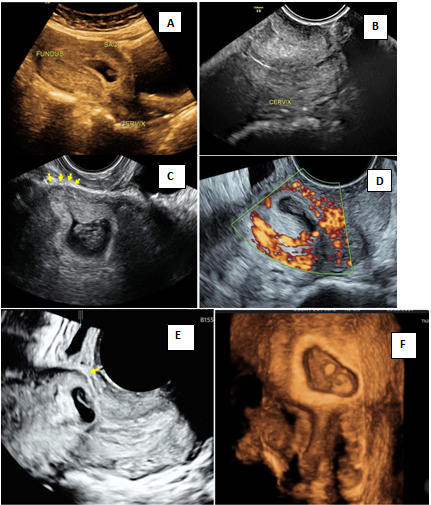
Figure 4 Caesarean scar pregnancy (CSP).
A) Lowlying gestational sac seen on transabdominal image B) Empty cervical canal in transvaginal examination C) Transvaginal image showing echogenic trophoblastic tissue in the scar site with anterior myometrial thinning (arrow) D) Peritrophoblastic flow on doppler E) 3D image showing anterior myometrial thinning (arrow) F) Rendered image showing empty cervical canal and sac implanted at scar site.
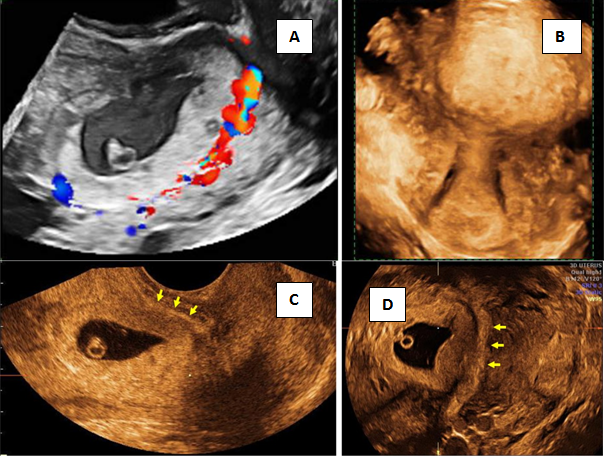
Figure 5 3D imaging in Caesarean scar pregnancy (CSP).
A) Peritrophoblastic flow B) Surface rendering mode of cervix and sac in CSP C&D) multiplanar imaging of gestational sac showing live embryo and yolk sac, sac implanted at the scar site with deficient anterior myometrium at bladder-uterine serosal interface (arrow).
Gestational sac that fills the niche of the scar with or without the presence of fetal pole and a negative sliding sign is in favour of CSP. Grading of CSP based on ultrasound findings are as grade I when the sac was embedded in less than one-half thickness of the myometrium. Grade II CSP is when sac occupied more than one-half depth of the implanted myometrium. In grade III CSP, the GS bulged out of the overlying myometrium and serosa and Grade IV indicated that the GS became an amorphous tumor with rich vascularity at the site of caesarean scar.33 MRI is an adjuvant tool for making a reliable diagnosis in cases of uncertain clinical and ultrasound features.34
Risk factors for MAP are advanced maternal age, multiparity, prior caesarean delivery, prior myomectomy, placenta previa, or previous uterine evacuation.35,36 With the increase in number of primary caesarean sections and prior uterine surgeries the incidence of MAP is increasing.37–40 Of the MAPs, placenta accreta occurs 75%, placenta increta 18%, and placenta percreta 7% of the time.35,39 Low lying gestation sac along with hypo echoic region and irregular placental- myometrial interface can also be a marker for placental invasive pregnancies.41
Sonographic biometric measurements were proposed by Moschos et al.42 to distinguish between abnormally adherent trophoblastic implantations and normally implanted pregnancies in first trimester.42 Figure 6 is an illustration of a low lying gestation with markers of abnormal placental invasion on ultrasound. The biometric measurements which were obtained were the distance from the external cervical os to the gestational sac (Figure 6D) and smallest distance from the anterior trophoblastic border to the uterine serosa (Figure 6E).
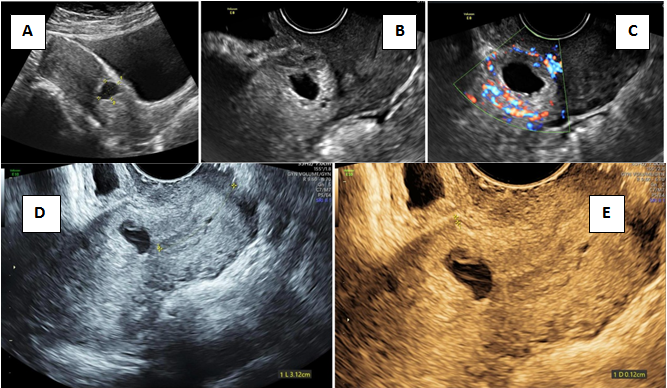
Figure 6 Low lying gestation sac.
A) Transabdominal image showing low lying gestation sac B) Transvaginal section depicting lack of anterior myometrial thickness C) Peritrophoblastic flow on colour D) Measurement of distance between inferior trophoblastic border and external os (3.12cm) E) Deficient anteriom myometrial thickness of 0.12cm.
Moschos et al.42 found that an anterior myometrial thickness of less than 5mm at the implantation site was equally predictive of abnormally adherent placentation. The association between first‐trimester smallest anterior myometrial thickness and histologically confirmed placental invasion at delivery was further studied and was observed that there was an inverse relationship between the myometrial thickness and the risk for placental invasion.43–46 Figure 7 is comparison of cervico isthmic implantation and CSP. Anterior myometrial thickness of 6 mm is noted in cervicoisthmic implantation (Figure 7A & 7B) and anterior myometrial thickness of 1mm is noted in CSP (Figure 7C & 7D).
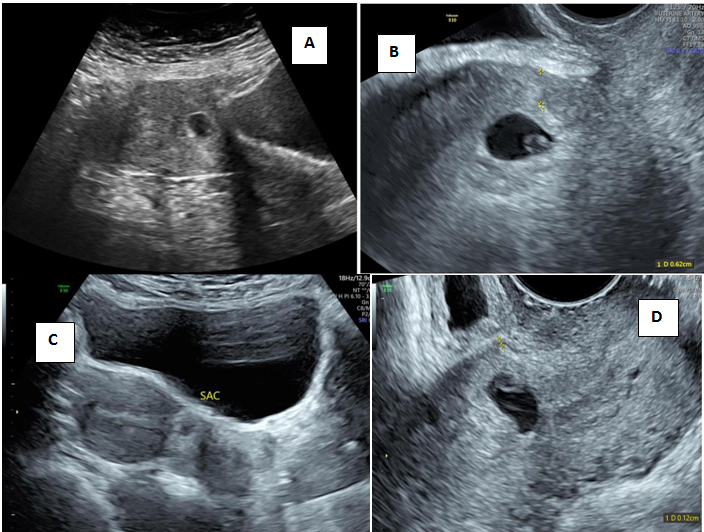
Figure 7 Comparison of anterior myometrial thickness in cervico isthmic implanation and CSP.
A&B) Case 1 low lying gestational sac with anterior myometrial thickness of 6mm C&D) Case 2 Low lying gestational sac with anterior myometrial thickness of 1mm.
Cross over sign (COS) has been proposed recently based on the relationship of gestational sac and the endometrial line on ultrasound to determine whether a CSP will progress to MAP. In sagittal view of uterus, a straight line is drawn connecting internal cervical os and uterine fundus through endometrium (endometrial line; long yellow line). Gestational sac is identified and superior–inferior (S–I) diameter perpendicular to endometrial line is traced (short red line) as shown in Figure 8. Superior-inferior (SI) diameter of the gestation sac was drawn perpendicular to the endometrial line and then categorized as COS-1 (Figure 8A & B) when at least two‐thirds of the S–I line was above the endometrial line, and as COS‐2 when less than two‐thirds of the S–I line of the gestational sac was above the endometrial line. Deficient myometrium in CSP with an anterior displacement of the sac more towards uterovesical interface tends to categorise as COS-1 criteria which has a poor prognosis.47
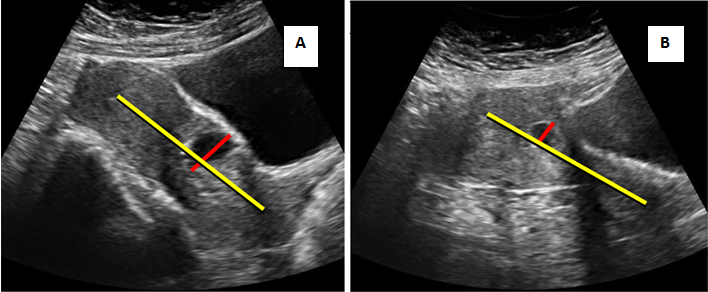
Figure 8 Cross over sign.
A & B) TAS - showing more than two thirds of SI Line above the endometrial line hence COS-1.
Figure 9 shows transabdominal and transvaginal images of central placenta praevia with large lacunae and abnormal placental vascularity and Figure 10 are a 3D illustration of MAP at 11-14 week scan. Figure 11 and figure 12 are two case scenarios of abnormal placental implantation. Figure 11 shows a placental invasive pregnancy presenting at 12 weeks of gestation with a normal looking fundus and cervix, placenta is seen implanted at LSCS scar site with deficient anterior myometrium at uterovesical interface at the level of cervical isthmus and has abnormal retroplacental vascularity. MAP at 11-14weeks scan if misdiagnosed as inevitable abortion leads to life threatening consequences. Figure 12 is an example of lowlying implantation of placenta which was referred for second opinion as a case of inevitable abortion. Ultrasound showed products of conception in the cervix and a fetal pole with absent cardiac activity (Figure 12A & Figure 12B). It is noteworthy to look at the closed external os (Figure 12C and implantation of the placenta at the level of the internal os with retroplacental vascularity (Figure 12D). Though the typical findings of cervical stage of miscarriage maybe present (compare 12e &f and 1c & d) the retroplacental vascularity and the appearance of closed external os shows that it is a case of low implantation of placenta in contrast to the diagnosis of cervical stage of miscarriage which has divergent modes of management.
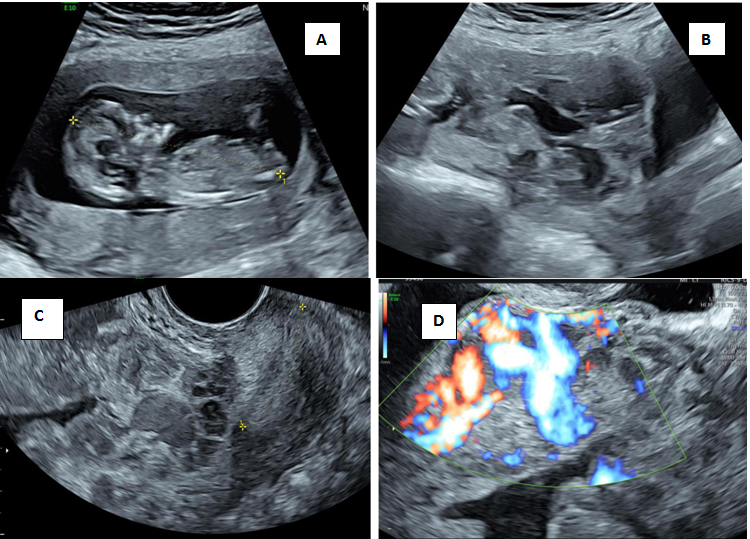
Figure 9 Morbidly Adherrent Placenta at 13weeks.
A) Crown Rump Length of 65mm corresponding to 13weeks B) Transabdominal image showing placenta implanted in lower uterine segment C) Transvaginal section showing placenta with lacunae covering internal os D) Abnormal retroplacental vascularity with intervillous flow.
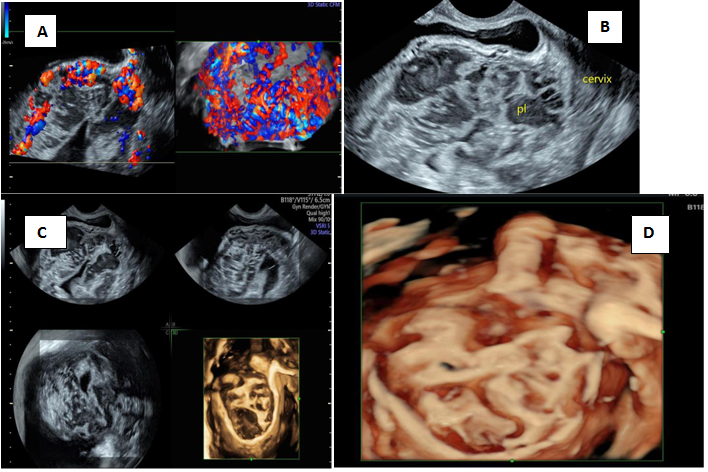
Figure 10 3D imaging of MAP in first trimester.
A) Retroplacental leash of blood vessels B) Placenta seen at bladder uterine serosal interface with no intervening myometrium C) Multiplanar rendering of placenta with lacuna D) HD live rendering of placenta with lacuna.
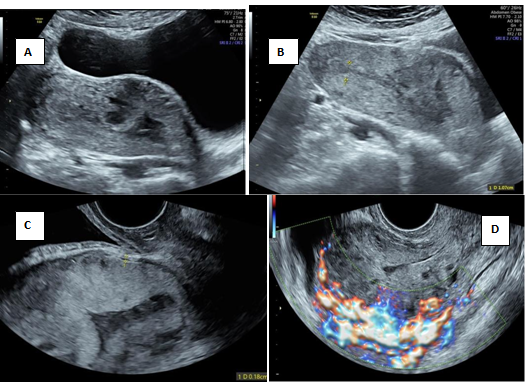
Figure 11 Low implantation at LSCS scar site.
A) Placenta implanted at LSCS scar site B) Normal looking fundus and cervix with placenta at the cervical isthmus C) Transvaginal section showing deficient anterior myometrium at bladder uterine serosa interface D) Abnormal retroplacental vascularity.
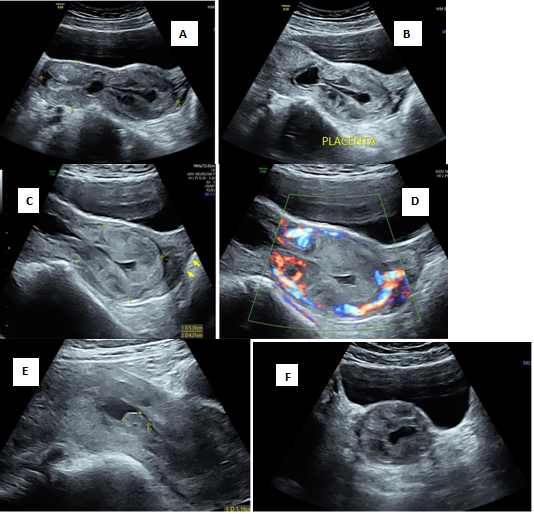
Figure 12 Low implantation of placenta.
A) Transabdominal section of uterus demonstrating hourglass appearance of cervix B) Fetal pole and absent cardiac activity C) Low implantation of placenta with intact external os (arrow to external os) D) Demostration of peritrophoblastic flow E) Intrauterine low lying gestation sac F) Transverse section of cervix showing the implanted placenta.
All cervico-isthmic implantations that do not fulfill the criteria for CP or CSP with an anterior myometrial thickness of greater than 5mm needs follow up for the possibility of MAP as shown in flowchart. (Figure 13) Application of the cross over sign further helps in predicting the probability of placental invasive pregnancy. Hence adopting a screening strategy based on ultrasound findings in early pregnancy scan during routine hospital visit can lead to the early accurate diagnosis of MAP.42–44 The major ultrasound findings in the second and third trimester consist of multiple large placental lacunae, abnormal placental vascularity and an ultrasound scoring system for MAP has been devised in midtrimester.48 The sensitivity and specificity for detecting placenta accreta in the first trimester were estimated to be about only 41% and 88% respectively and this reiterates the need for subsequent monitoring in mid and third trimester in suspicious cases.49
Timor et al in their review concluded that CSP and placenta accreta are a difficult diagnosis and often misdiagnosed as low intrauterine pregnancy, cervical pregnancy or miscarriage in progress. The best diagnostic tool in early identification of this entity is high frequency transvaginal ultrasound at the early pregnancy scan .The earlier the diagnosis the better the outcome, and it starts with patient education. The patient after caesarean delivery should be advised on an early visit to the obstetrician in future pregnancy.21 Placenta accreta is associated with high maternal mortality of up to 10% and morbidity of about 75%, including uterine rupture before viability, massive hemorrhage, multiorgan failure and need for hysterectomy.51 As placenta accreta is now the most common reason for caesarean hysterectomy, it is ideal to screen for MAP at the early pregnancy scan itself.52–54 For patients who choose to continue with pregnancy, early diagnosis aids in prompt referral to a tertiary centre.
Women presenting with bleeding PV and low lying gestational sac in early pregnancy needs a meticulous ultrasound evaluation to arrive at a correct diagnosis. It is essential for every clinician to watch out for CSP in women who conceive after caesarean, and actively involve the patient in decision making after discussing the available treatment modalities. Ultrasound has an important role and meticulous attention to the subtle markers helps to differentiate the benign conditions from life threatening ones. The diagnosis of cervical ectopic and caesarean pregnancy should not be missed as the maternal mortality due to haemorrhage is high in these conditions. Early diagnosis of a MAP allows providers to coordinate a multidisciplinary treatment approach and thoroughly counsel patients for delivery at tertiary care centre.
None.
Consent was obtained from all patients included in the study. This is only an observational study.
None.
The author and co-authors have no conflicts of interest relevant to this article.

©2020 Lakshmy, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.