eISSN: 2377-4304


Case Report Volume 9 Issue 5
1Department of Obstetrics and Gynecology, Southern California Kaiser Permanente, USA
2St. George's University, School of Medicine, Grenada
3Maternal Fetal Medicine, Department of Obstetrics and Gynecology, Lincoln Medical and Mental Health Center, USA
Correspondence: Shadi Rezai MD, Department of Obstetrics and Gynecology, Southern California Kaiser Permanente, Kern County, 1200 Discovery Drive, Bakersfield, California, 93309, USA
Received: July 04, 2018 | Published: September 28, 2018
Citation: Rezai S, Kochen D, Patel ND, et al. Long-term fertility-sparing, conservative management of endometrial cancer with progestin, a case report and review of literature. Obstet Gynecol Int J. 2018;9(5):338-342. DOI: 10.15406/ogij.2018.09.00360
Background: In premenopausal women, endometrial cancer presentation may be asymptomatic or present as abnormal uterine bleeding. The gold standard treatment for endometrial cancer is total hysterectomy, bilateral salpingo-oophorectomy, pelvic and para-aortic lymphadenectomy with peritoneal cytology. However, this definitive treatment eliminates childbearing for young reproductive age females. Confirmation of a low-grade, well differentiated, Type 1 cancer, confined to the endometrium without myometrial invasion or spread; supplemented with MRI to rule out metastasis may benefit from medical management. Medical management includes hormone therapy primarily with progestin analogs.
Case: 42-year-old, Gravid 0, female with last menstrual period in February 2018 was evaluated for menorrhagia and irregular menstrual bleeding for 3 months. Her past medical history was significant for two cases of endometrial carcinoma (2006-2007, 2008-2011) treated with progestin therapy.
In 2006, the patient was diagnosed with early-stage endometrial cancer and declined hysterectomy to preserve her fertility. She was treated with progestin and returned in 2007 showing remission of endometrial cancer. In 2008 she presented again with irregular vaginal bleeding; work-up revealed Grade 1 endometrial cancer with squamous differentiation and progesterone effect; patient was treated with progestin.
Subsequently, follow-up dilation and curettage in 2011 revealed no cancer present.
In May 2018, an endometrial biopsy performed for irregular bleeding, showed endometrial hyperplasia with atypia suspicious for adenocarcinoma; endocervical curettage and endocervical polyp showed similar pathology. In the meantime, the patient was started on progestin therapy. She ultimately underwent hysterectomy and bilateral salpingo-oophorectomy with no complications. The patient declined the gold standard treatment of hysterectomy, in order to preserve fertility. Alternatively, the patient underwent progesterone treatment and remained in early-stage endometrial cancer for a 10-year period. Unfortunately, the patient never conceived.
Conclusion: Fertility preservation has always been a priority for many reproductive and perimenopausal age women. This juxtaposes the most effective treatment modality, hysterectomy, for patients who desire fertility preservation. Progestin and progestin derivative medical management now gives reproductive age females an opportunity to combat endometrial cancer, while retaining the ability to conceive. However, the risk of resistance and recurrence still remain with medical management.
Keywords: conservative, fertility-sparing, hormonal therapy, endometrial cancer, megace, Megestrol, progestin
Endometrial cancer is the most common cancer of the female reproductive organs, the American Cancer Society estimates 63,230 new cases in 2018 alone.1 Although endometrial cancer is typically a disease affecting post-menopausal women, a small percentage of woman are diagnosed before the age of 40.2 These patients can present without any symptoms or with abnormal uterine bleeding characterized by intermenstrual bleeding or prolonged menstruation.
Uterine cancer is classified into two main subtypes. Type 1 consists of endometrioid cells that are commonly low grade, well differentiated, and typically hormone receptor positive. Risk factors for Type 1 are associated with unopposed estrogen: early menarche, late menopause, tamoxifen use, nulliparity, infertility, failure to ovulate and polycystic ovarian syndrome (PCOS). In addition hypertension, diabetes and obesity also increases the risk of developing endometrial cancer.3 Type 1 cancer has a favorable prognosis since the likelihood of metastasis is low, and the cancer is typically defined to the uterus.3 Type 2 cancers are common in non-obese females, typically high grade, poorly differentiated, does not have hormone receptors, and carries a poorer prognosis since it tends to metastasize.3
The gold standard treatment for endometrial cancer is total hysterectomy, bilateral salpingo-oophorectomy, pelvic and para-aortic lymphadenectomy with peritoneal cytology. This eliminates childbearing for young, reproductive age females.3,4 Women who desire a fertility sparing option may undergo medical management; keeping in mind there is a risk of recurrence.5 Patients confirmed to have a low-grade, well differentiated, Type 1 cancer, confined to the endometrium without myometrial invasion or spread supplemented with MRI to rule out metastasis may benefit from medical management.4 Medical Management includes hormone therapy with: oral progestin analogs such as medroxyprogesterone acetate (MPA) or megestrol acetate (MA), progestin releasing IUD, selective estrogen receptor modulators, aromatase inhibitors, gonadotropic releasing agonists and/or retinoid derivatives.2,6,7,8,9
We present a case report and literature review in which progestin therapy is used for long-term fertility-sparing endometrial cancer management.
42-year-old, Gravid 0, female with last menstrual period in February 2018 was evaluated for menorrhagia and irregular menstrual bleeding for the past 3 months. Her past medical history was significant for two cases of endometrial carcinoma (2006-2007, 2008-2011) treated with Megace and Provera, respectively; and Type 2 Diabetes Mellitus; patient does not smoke or consume alcohol; family history is significant for Type 2 Diabetes Mellitus, patient denies any family history of breast, ovarian, or colon cancer. Past surgical history includes dilation and curettage (2006, 2007, and 2011), dilation and curettage with laparoscopy, right ovarian cystectomy and pelvic node biopsy (2009). Upon presentation, vital signs were stable and within normal limits; BMI 34.16 kg/m².
In 2006, at the age of 30, the patient was diagnosed by an alternate provider with early-stage endometrial cancer with no myometrial invasion and declined hysterectomy to preserve her fertility. She was treated with Megace, in 2007 end of treatment dilation and curettage showed remission of endometrial cancer. Patient was instructed to discontinue Megace. In 2008 she presented again with irregular vaginal bleeding to the same facility; endometrial biopsy showed Grade 1 endometrial cancer - patient was started on Provera for treatment. Two months later, patient underwent dilation and curettage with laparoscopy pelvic node biopsy and right ovarian cystectomy for persistent irregular vaginal bleeding. Pathology revealed grade 1 endometrial cancer with squamous differentiation and progesterone effect confined to the uterus; patient was treated with Megace 40-mg daily, for a total duration of 3 years. Due to patient non-compliance and incomplete follow up dilation and curettage could not be preformed every 3 months. Subsequently, follow-up dilation and curettage in 2011, showed decidual effect, with no cancer present, ovulation induction cycling was initiated with no success. Patient continued Megace until 2013, then discontinued.
Patient was lost to follow-up until she presented in May 2018 for irregular bleeding and endometrial biopsy showed endometrial hyperplasia with atypia suspicious for adenocarcinoma; endocervical curettage and endocervical polyp showed similar pathology. Pap screen and HPV were negative for intraepithelial lesion or malignancy; CA-125 was 43.4. Pelvic sonogram showed the uterus (11.3 by 7.6 by 8.5) with an endometrial mass (10.5 by 5.8 by 6.6) (Figure 1). In addition, there was a 19-mm cyst in the right ovary (Figure 1). On review of the sonogram, the endometrial mass is heterogenous and extended into the endocervical os (Figure 1). Concurrent chest x-ray was within normal limits with no abnormalities; CT of the chest, abdomen, and pelvis (Figure 2) was negative for chest and liver metastasis and hydronephrosis; positive for 7-mm paraaortic node, 9-mm pelvic node, right ovarian cyst (2.8-cm), and an endometrial mass (6.7 by 6.4-cm). Physical exam showed midline cervix 2.0 cm in diameter, soft but with 4 by 5 mm ass protruding from the cervical os. Uterus is 12 by 10 by 8 cm anterior not tender. Adnexa were not appreciated and no nodularity in the cul de sac. The patient was started on Megace 40mg daily. At this time, hysterectomy, bilateral salpingo-oophorectomy and staging were indicated.
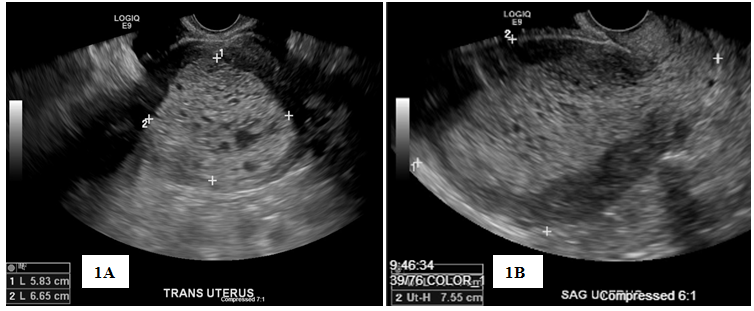
Figure 1 Pelvic Ultrasound, Transverse view (1A) and Sagittal view (1B) showing endometrial mass measuring 10.5×5.8×6.6cm, highly suspicious for endometrial neoplasia.
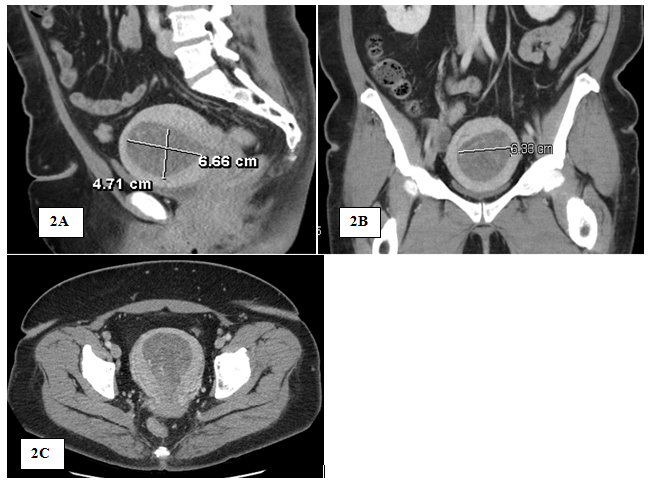
Figure 2 Computed tomography (CT) of Pelvis: sagittal View (2A); Coronal View (2B); and Transverse View (2C), showing 6.7×6.4×10cm hypodense mass in the endometrial cavity compatible with known uterine cancer.
The patient underwent hysterectomy and bilateral salpingo-oophorectomy in May 2018. Her post-operative course was uncomplicated and uneventful. She was able to tolerate regular diet, ambulate, pass flatus, and void on her own. Pain was well controlled and was afebrile with vital signs within normal limits. Incision site was clean, dry and intact and patient was discharged post operation day 2 with follow up at the gynecology clinic. Pathology from hysterectomy showed Grade 1 endometrial carcinoma with less than 1mm of myometrial invasion.
To summarize, the patient declined the gold standard treatment of hysterectomy, in order to preserve fertility. Alternatively, the patient underwent progesterone treatment and remained in early-stage endometrial cancer for a 10-year period. Unfortunately, the patient never conceived.
Endometrial cancer is a disease of postmenopausal women; diagnosis under the age of 40 is considered to be an unusual presentation and tend to have more favorable outcomes compared to older patients.10 Diagnosis of cancer is established through dilation and curettage or endometrial biopsy, supplemented with MRI to evaluate for invasion.
Hysterectomy with bilateral salpingo-oophorectomy with or without lymphadenotomy is the gold standard treatment. Medical management is an alternate option for early stage cancer for reproductive age women who desire to conceive.3 Fertility sparing treatment using progesterone is effective for early stage, well differentiated, endometrioid type cancer with no evidence of extra uterine spread.11,2 Progesterone therapy has shown favorable therapeutic results for conceiving and successfully carrying a normal pregnancy, however the risk of recurrence still remains, even after progesterone therapy is completed.12,13,14
There are four commonly used progesterone medications combinations prescribed for conservative management of endometrial cancer. Table 1 demonstrates these treatment options, with associated success rates, pregnancy rates, and live birth rates.15 Studies have shown that the use of oral or intrauterine progestin is equally effective for fertility sparing management of complex atypical endometrial hyperplasia.6,14,16−18 The average duration of hormonal therapy is approximately six months; with an average response time of twelve weeks. 76% of patients treated with hormonal therapy had a complete response and the other 24% never responded to treatment - Of those who initially responded, 66% percent did not show recurrence of disease; the other 34% had a relapse.10 One report supports increasing the dose if the initial response is not achieved, or when disease recurs.19 Serious complications were not observed with the use of higher doses.9,19 Upon initiation of therapy, follow up screening and surveillance through dilation and curettage, every 3 months is recommended for evaluation.3,11
Drug |
Success rate |
Pregnancy rate |
Live birth rate |
Medroxyprogesterone acetate (Provera) oral |
71% (63-77) |
34%(30-38) |
20% |
Megestrol Acetate (Megace) oral |
71% (63-77) |
34%(30-38) |
20% |
Progestin IUD |
76 (67-83) |
18% (7-37) |
14% |
Progestin oral + IUD |
87% (75-93)) |
40% (20-63) |
35% |
Table 1 Comparison of success rate, pregnancy rate and live birth rate of four progestin interventions used for early stage endometrial cancer22
Progesterone treatment can begin to have an effect on endometrial tissue as early as 10 weeks from initiation. Therapy follow-up should be done every 3 months in order to assess for pathologic response until complete response is achieved.5 Increased use of hysteroscopic biopsy is not recommended because of repeated damage to the basal layer by thermal injury, fibrosis, and trauma will decrease the success of pregnancy in the future. Patients who desire to conceive immediately after treatment should undergo ovulation induction once progestin effect is seen on histology. Patients, who want to delay pregnancy, should be placed on low dose cyclic progestin or progestin IUD to counteract the unopposed estrogen until they wish to conceive.5 After completing family planning, a prophylactic hysterectomy should be advised due to the likelihood of recurrence. Patients with recurrent disease, who have not achieved a successful pregnancy are able to undergo progestin retreatment since the histology of these tumors are typically well differentiated, however; treatment outcomes have not been well studied.5 If metastasis is suspected, hysterectomy along with chemotherapy is suggested.
Over time, patients may experience resistance to progestin therapy alone – up to 30% of women.8 New combination therapies have been introduced in attempt to overcome the resistance. A case presentation proposed the use of MPA and anastrozole daily for 3 and 6 months respectively for reversion to normal endometrium.7 Progesterone combined with the elimination of adipose estrogen production was effective for differentiated endometrial cancer, especially in obese pre-menopausal women.7 Another combination trial consisted of MA with Tamoxifen and Temsirolimus. However, while this combination did not show enhanced activity, or improve resistance, it was associated with an increased risk for venous thrombosis.20
Advancements in progesterone therapy are being studied to optimize medical management. A fourth-generation progestin, Dienogest, provides a fertility sparing alternative to patients who have previously failed to respond to MPA.21 Dienogest antitumor effects work by suppressing proliferation of endometrial cancer cells in-vitro; and is used for endometriosis-associated pain.21 Additional non-progesterone therapies include anti-cancer retinoid analogs.
The anti-cancer effect of Fenretinide, a retinoid derivative, was found to decrease tumor size and have an anti-tumor effect against endometrial cancer.8 Fenretinide acts to decrease cell viability, increase apoptosis, and cause a decrease in tumor size. It is postulated that Fenretinide induces apoptosis because of an increase in retinol uptake.8
Fertility preservation has always been a priority for many reproductive age women. Even though endometrial cancer primarily affects post-menopausal women, it is seen in younger premenopausal women. Treatment maintaining fertility juxtaposes the most effective treatment modality, hysterectomy. Progesterone therapy gives reproductive age females a non-surgical treatment option, while retaining the ability to conceive in the future. However, medical management is not a wide-spread standard treatment opportunity; rather it is evaluated on individual circumstances, taking into consideration the aggressiveness and staging of the cancer. Progestin therapy has shown to impact endometrial growth at all stages; endometrial hyperplasia has highest likelihood of favorable response, followed by Grade 1 endometrial carcinoma at 66% and 48% respectively. However, the risk of resistance and recurrence still remain after completion of medical management for endometrial cancer.22
None.
Authors did not report any potential conflicts of interests.

©2018 Rezai, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.