eISSN: 2377-4304


Case Report Volume 9 Issue 1
1Department of Obstetrics and Gynecology, American British Cowdray Medical Center, Mexico
2Department of Surgical Oncology, American British Cowdray Medical Center, Mexico
3Department of Gynecologic Oncology, American British Cowdray Medical Center, Mexico
Correspondence: Milagros Pérez Quintanilla, Department of Gynecologic Oncology, American British Cowdray Medical Center, Av Carlos Graef Fernÿndez, 154 Gynecology tower, 3rd 342 Office Floor, Mexico, Tel 52 55-5272-3324
Received: December 20, 2017 | Published: February 7, 2018
Citation: Carrera JH, Gomez D, Rocha EB, et al. Liposarcoma of the breast after phyllodes tumor resection: case report. Obstet Gynecol Int J. 2018;9(1):46-47. DOI: 10.15406/ogij.2018.09.00302
Phyllodes tumor (PT) of the breast are neoplasms typically presented as painless, lobulated with a size that varies from 2 to 10 cm, they have an incidence of less than 1% of all breast tumors. We present the case of a 75-year-old woman with a history of a benign fusocellular tumor of the right breast diagnosed with a borderline phyllodes tumor and then presenting degeneration to a liposarcoma.
Keywords: Breast cancer, Liposarcoma, Phyllodes tumor, Rare malignancies, Soft tissue sarcoma
Phyllodes tumor (PT) of the breast are neoplasms presented as painless, lobulated with a size that varies from 2 to 10 cm, the incidence is less 1% of all breast tumors. Histologically, they can be benign, borderline or malignant fibroepithelial neoplasms being the malignant type the most uncommon of all,1 display a biphasic growing pattern leaf-like glandular of luminal and myoepithelial cells.1 The epithelial component may contain hyperplasia without atypia or metaplasia with apocrine or squamous components. These tumors have significant heterogeneous tissue and genes that have an impact role in the malignant progression of PT including CDKN2A, HOXB13, PAX3, SIX1, HMGA3, and TGFB2.2-5
Evaluation consists in stromal cellularity, nuclear atypia, mitotic activity, stromal overgrowth, and tumor margin appearance, classified into benign, borderline and malignant. Lipoma is the most common lipomatous tumor arising in the breast. Tumor grade and margin status have been reported to be associated with the risk of local recurrence with the risk of local recurrence (17-27%).5
Recurrences typically develop within 2-3 years. Distant metastasis occurs in 22%.6 Microscopically, the stromal component resembles fibroadenoma, a sarcoma, or it can even vary between these two types.7 Liposarcomas have different presentations like pure primary liposarcoma or cystosarcoma phyllodes.
The malignant stroma seen in PT often differentiates into sarcoma but rarely into heterologous tissue. Other includes angiosarcoma, leiomyosarcoma, chondrosarcoma, osteosarcoma and rhabdomyosarcoma.8-10 Fat containing hamartomatous lesions have been recognized by many names including adenolipoma4 and fibroadenolipoma.5
A 75-year-old woman with a history of a benign fusocellular tumor of the right breast, radius 6 (R6), resected on December 2006 with histopathological result as a myofibroblastoma of the breast, size 2.1 mm, with a component of ductal hyperplasia; presented with a nodular lesion at the same previous location (R6) and with a mammogram test BIRADS 4C. On June 2016, she had mammogram test BIRADS 2, physical examination normal and 6 months after with a history of approximately 2 months feeling discomfort, pain and redness.
Physical exam showed the right breast skin ticked on inferior inner quadrant, and a 3 cm nodule in the axillary tale. The tissue underlying the R6 scar was higher in density along with the breast in the inferior inner quadrant. Her results were BIRADS 5 with arquitectural distortion over surgical scar. Breast Ultrasound: thickening of the skin in the internal inferior quadrant with high density and nodular aspect 1.38 cm, R9 nodular lesion size: 2.88 cm compatible with lymph node with eccentric hilum). The case was reviewed with the breast image department and core biopsies were taken from the arquitectural distortion reported. The R9 nodule core biopsy was reported as tissue with sarcomatoid changes probably sarcoma versus high-grade PT (Figure 1).
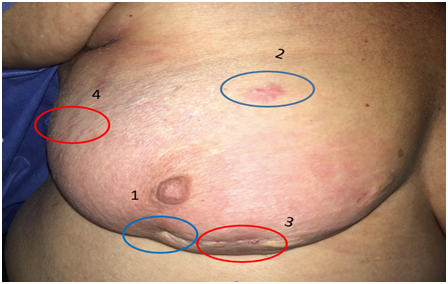
Figure 1 Axillar tale nodule:
The patient was programmed for resection of the nodule and skin biopsy around right breast R3-R4. The final pathology report was:
Modified radical mastectomy (MRM) was performed with pathology report of low-grade periductal stromal sarcoma; we referred the patient to the MD Anderson Cancer Institute Sarcoma Service, because of the low incidence and rarity of this pathology. The case was discussed and the patient received radiotherapy and was subsequently discharged. Last PET-CT study of June 2016 without tumor activity data.
Liposarcomas of the breast may occur either as pure primary liposarcoma or arise in cystosarcomas phyllodes. The patient presented with a breast tumor that was a malignant PT with heterologous liposarcomatous differentiation.8
The atypia of the stromal cells in a lesion considered benign at first should include in the differential diagnosis the possibility of a PT. The excisional biopsy was histologically diagnosed as malignant PT with liposarcomatous components. Peculiar atypical cells with pale, large transparent cytoplasm normally guide obtains a diagnosis. There is little information about patient follow up and recurrences and metastasis after surgery for this rare breast tumor. Further investigation is needed.
PT is commonly a benign neoplasm that in some rare cases may differentiate to a malign entity which diagnosis is often difficult and it’s considered as an exclusion diagnosis. In the case of our patient, multiple procedures were done in the same mammary region with a first result of phyllodes tumor and then presenting degeneration to a liposarcoma. The treatment for this rare condition is to perform a MRM with radiotherapy, normally with excellent survival rate. Currently the patient is without evidence of tumor activity.
None.
The authors declare that they don’t have any conflict of interests.
Patient’s consentment was given to us in order to publish this case report.

©2018 Carrera, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.