eISSN: 2377-4304


Case Report Volume 9 Issue 6
1Obstetrician-gynecologist, New Granada Military University- Gynecology and Obstetrics Unit, University Hospital San Rafael Clinic, Colombia
2Pediatrician, New Granada Military University, University Hospital San Rafael Clinic, Colombia
3Specialist in Maternal-Fetal Medicine, Gynecology and Obstetrics Unit, University Hospital San Rafael Clinic, Colombia
4Specialist in Pediatric Cardiology-Hemodynamics, Pediatric Unit, University Hospital San Rafael Clinic, Colombia
Correspondence: Diana Cecilia Poveda-Rojas, Obstetrician-gynecologist, New Granada Military University- Gynecology and Obstetrics Unit, University Hospital San Rafael Clinic. Bogotÿ, Colombia
Received: December 17, 2018 | Published: December 26, 2018
Citation: Poveda-Rojas DC, Vélez-Tirado N, Bonilla-Cortes L, et al. Fetal complete atrioventricular block: diagnostic and therapeutic approach: a case report and review of the literature. Obstet Gynecol Int J. 2018;9(6):526-530. DOI: 10.15406/ogij.2018.09.00401
Objective: To report a case of congenital complete atrioventricular block and perform a literature review of diagnosis and treatment.
Materials and methods: The case of a pregnant 27 year old who consults a general hospital of high complexity, with a pregnancy of 33 weeks single fetus diagnosed with complete atrioventricular block and dilated cardiomyopathy secondary is reported. Prenatal management began with Betamimetic with poor response so it was necessary to end the pregnancy. The newborn requires implantation of ventricular pacemakers in the first day of life with excellent results in follow-up to 1 year. Medline, Lilacs, SciELO, by the words: literature review published in the data bases is done "fetal complete atrioventricular block", "congenital complete heart block" with limits year from 2000 to 2016, Spanish and English.
Results: 21 publications were obtained; seven case reports ten literature reviews four cohort studies. The diagnosis is based on fetal echocardiography showing valvular regurgitation, myocardial/valvular hyperechogenicity - endocardial fibroelastosis, premature atrial contractions and pericardial effusion. Prenatal treatment with respect to the corticosteoides and Betamimetics, are the most commonly used medications. Treatment of severe refractory neonatal bradiarritimia may require implantation of a pacemaker as definitive management
Conclusion: Congenital third-degree AV block requires early diagnosis and treatment is associated with high perinatal morbidity and mortality. More studies are needed methodological quality that allow other options to endorse promising therapeutic regimens.
Objective: To present a case of congenital atrioventricular block and a complete review of the literature.
Materials and methods: A congenital complete atrioventricular block and dilated cardiomyopathy secondary in a 27-year-old woman is Reported. Prenatal management was begun with beta-adrenergic agonists, but the pregnancy was terminated by caesarean section in the 37th week due to maternal cholestasis. An epicardial ventricular pacemaker was implanted to the newborn on the first day of life with excellent results. A review of the literature published MedLine, Lilacs, and SciELO, was made using terms "fetal complete atrioventricular block", "congenital complete heart block"; With year from 2000 to 2016 limits, Spanish and Inglés. We also included in the literature review Up to Date with the term "Neonatal Lupus" and the review based on a book chapter.
Results: Twenty references, seven are case reports, literature reviews are nine, and four retrospective cohort studies, two prospective and two retrospective cohort studies.
Conclusion: Congenital AV block third-degree is rare, but with high perinatal mortality and morbidity, early diagnosis and which requires timely treatment. Studies are higher methodological quality required to support promising therapeutic options and schemes.
Keywords: bradyarrhythmia, atrioventricular block, systemic lupus erythematosus, atrioventricular block, bradycardia, lupus erythematosus, systemic
Fetal bradyarrhythmia is defined as the presence of heart rate below 110beats per minute (lpm) for 10 minutes in the absence of fetal distress.1 Fetal bradyarrhythmias include sinus bradycardia, extrasystole not conducted headphones associated couplets atrioventricular block of second and third degree; It not included in this classification the atrioventricular (AV) block first degree not to cause bradycardia. The difference between each of the types of AV blocks lies in the relationship between the atrium and ventricle; in the first degree block the PR segment is extended in all atrial impulses conducted to the ventricle successful manner; AV block in the second degree, PR segment extends with each beat until there is an atrial pulse that fails to be transmitted to the ventricle and finally in the AV block grade III no evidence atrioventricular dissociation.2 The heart rate can help determine the etiology of the lock,3 has been the frequency 80-100lpm is common in sinus bradycardia; frequency lpm 60-80 is more common in the AV block grade II and III, like in the presence of extrasystoles not conducted headphones associated couplets; finally less than 60bpm heart rate almost always associated with third degree AV block.3 The heart rate can help determine the etiology of the lock,3 has been the frequency 80-100lpm is common in sinus bradycardia; frequency lpm 60-80 is more common in the AV block grade II and III, like in the presence of extrasystoles not conducted headphones associated couplets; finally less than 60bpm heart rate almost always associated with third degree AV block.3 The heart rate can help determine the etiology of the lock,3 has been the frequency 80-100lpm is common in sinus bradycardia; frequency lpm 60-80 is more common in the AV block grade II and III, like in the presence of extrasystoles not conducted headphones associated couplets; finally less than 60bpm heart rate almost always associated with third degree AV block.3
It has been reported that constitute 2% of all fetal arrhythmia, being 50% caused by congenital heart (left atrial isomerism, transposition of great arteries and endocardial), and 50% of immunologically associated with connective tissue diseases maternal.1,4,5 Regarding immunological origin, this is caused by anti-Ro, and anti-LA maternal antibodies in 85-90%, and these antibodies expressed in collagen diseases such as systemic lupus erythematosus and Sjögren's síndrome.6 A percentage of maternal carriers of anti-Ro and anti-La no symptoms of the disease, so there is a group of women who will be pregnant without knowing the risk to the fetus;7 fortunately only about 2% of seropositive mothers fetuses present heart condition, particularly the piping system.5,7 To understand the pathophysiology of AV congenital blocking is necessary to understand the fundamental role of anti-Ro and anti-La antibodies, which are directed against antigens ribonucleoprotein complex present in the cells of cardiac conduction and cardiac myocytes located in the cytoplasm.8 IgG binding to these antigens triggers immunoglobulin three pathological mechanisms:
The antibody may be transferred at any time in gestation,10 initiating its placental passage 12 weeks, the period of greatest vulnerability ranges from 16 and 24 weeks generally having a peak in antibody concentration, which explains the fact that over 80% of cases of congenital AV block are diagnosed before week 30.4,10
The prognosis when associated with congenital heart disease is unfavorable, reaching a mortality rate of 50-80% in the 1st year of life, being left atrial isomerism malformation that confers higher mortality.11 This contrasts with blocking immunologically whose intrauterine mortality is 6%, 16-19% overall mortality and when associated with endocardial fibroelastosis of 69%, requiring the use of pacemakers up to 70% of cases during the first 10 years of life.11,12 The risk of recurrence in future pregnancies is of 10-17.4%.13
Being a rare entity is important early recognition and timely management by the obstetrician looking to improve the outcome of the newborn, so the case is presented a pregnant pursuing a pregnancy in which the fetus is a carrier of AV block third grade, requiring early pacemaker implantation in the newborn; in order to conduct a review of the literature which seeks to highlight the most important aspects of diagnosis and treatment.
Patient 27, G2A1V0, who was admitted with gestational age of 33 weeks at the obstetrics unit at University Hospital Clinic San Rafael (HUCSR), institution fourth level of complexity, located in Bogotá, serving patients at high maternal fetal risk belonging compulsory health plan. The patient was referred for outpatient fetal bradycardia and AV block suspected diagnosis of fetal grade II from week 25 of gestation for assessment and management of maternal-fetal medicine and pediatric cardiology.
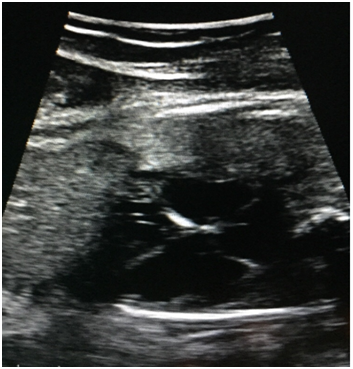
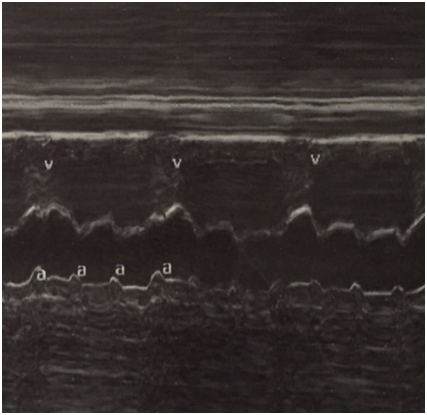
On admission to HUCSR fetal well tests which are performed are normal, then ultrasound detail in which no signs of fetal hydrops or other anatomical abnormalities observed is performed. Finally fetal echocardiogram where situs solitus, normal vascular situs, cardiac shaft 45, cardiothoracic ratio 0.55, cut normal four chambers, atrioventricular and ventriculoarterial accordance normal valve insertion, patent foramen ovale, flow to the left atrium, movements walls is performed free ventricles of normal amplitude, hyperechoic observed level suggestive sinoatrial node endocardial fibroelastosis (Figure 1). No evidence sinus rhythm, atrioventricular dissociation documented in M (Figure 2) Mode, with ventricular heart rate 50bpm and 135bpm atrial rate, PR segments variables, aortic isthmus with antegrade flow. There is no evidence of diastolic dysfunction given normal venous ductus.
immunological profile are requested to mother and operation starts with dexamethasone 4mg orally daily. report positive antinuclear antibodies (ANAs) 1/80 with speckled pattern thus diagnosing lupus-like syndrome is is received. Extension studies reported lupus anticoagulant negative, anti Smith (anti-SM) and anti ribonucleoprotein (RNP anti-) negative, positive anti-Ro, anti-La negative. Subsequently it is present marked fetal bradycardia (less than 60bpm) joined findings make atrioventricular dissociation is considered that the patient has progressed degree atrioventricular block III. Oral terbutaline 5mg every 4hours starts, the mother is hospitalized to monitor lung maturation and biophysical profile with every 48hours looking achieve 2500g reach the fetus. After the start of terbutaline FHR 86lpm is achieved, however, by the presence of severe headache, tachycardia and tremor in the mother is necessary to decrease the dose to 2.5mg every 6hours via oral. Given persistent low fetal heart rate, presence of dilated cardiomyopathy and appropriate weight for gestational age 37 weeks gestation end is decided by elective cesarean.
In the operating room it is obtained newborn female, vigorous, with appropriate size and weight for gestational age; Ballard 38 weeks; with suitable adaptation neonatal despite having heart rate 50bpm. Initially no signs of low cardiac output or respiratory distress. About 12hours old newborn having difficulty breathing, filling slow capillary prone to hypotension by which it is decided to initiate invasive mechanical ventilation, attempts to improve heart rate with pharmacological measures, however the patient does not respond; Chest radiography (Figure 3) showing cardiomegaly taken. Cardiovascular surgery who perform scheduled ventricular pacemaker epicardial unicameral are reported, with heart rate of 120pm and 0.9 threshold. After surgery the newborn is transferred to the intensive care unit pediatric to continue comprehensive management. In echocardiogram ventricular chamber pacemaker demand (VVI) with a suitable structurally sound and biventricular heart function is evident. The evolution of the newborn year of life has been satisfactory, with proper neurological development and weight.
Medline via PubMed, Lilacs and SciELO, using the terms: a review of the literature published in the databases was performed "fetal complete atrioventricular block" and "congenital complete heart block", "congenital heart disease" The search was limited to published between 2000 and January 2017, with English or Spanish jobs. Reports, case series, cohort studies and literature review were included. The review was also conducted on Up to Date with the term "Neonatal Lupus".
Ethical aspects: verbal and written consent was requested (by informed consent) for the publication of the case and the use of photographic archive. Information confidentiality and anonymity of the patient is guaranteed.
34 publications described terms and limits, which are selected 20. Of these, seven publications correspond to case reports with literature review, conducted in the United States4,5 were obtained; Colombia;10 Germany;14 Canada and the United States;15 Australia16 and Italy.17 The other 14 selected publications covering 9 literature reviews2,3,18–22 and five cohort studies being 3 retrospective8,11,13 and two forward.9,12 Their approaches were in prognosis11,13 and diagnosis and prognosis8,9,12 were made in the United States,8 Italy,9 Finland,11 Canada12 and the USA.13
Diagnosis
The most common method for prenatal diagnosis was fetal echodopplercardiogram with the use of M and pulsed Doppler to determine the PR interval mode, atrioventricular relationship and detecting intracardiac anomalies.2,3,4 Within the fetal echocardiographic findings documented Matta et al in a review of the literature report that valvular regurgitation was the most common finding (63.2%); followed by myocardial hyperechogenicity/valvular- endocardial fibroelastosis in 10.5%, the premature atrial contractions at 7.9% and finally pericardial effusion by 2.6%.2 These alterations are developed in 1-2% of cases AV block third degree and produce irreversible injury reported.8–10
A narrative review15 suggests that once documented fetal bradyarrhythmia is important to evaluate the characteristics of the arrhythmia and determine if there is hemodynamic compromise also proposes an echocardiogram every 8 days between weeks 16 to 28, should be normal It must be repeated from week 28 every 2-4 weeks.
Treatment
Fluorinated steroids such as betamethasone and dexamethasone cross the placental barrier, enter the fetal circulation and alleviate cardiac inflammation caused by maternal antibodies improving cardiac contractility;14,15,20,21 so they are best used to grade I and II blocks according to a review of the literature.20 However, the same review notes that this group of drugs used increasingly less because of reports of adverse effects as fetal adrenal insufficiency, and neonatal and altered growth and neurodevelopmental. Weber et al recommend dexamethasone 8mg/day for two weeks then the dose is reduced to 4mg/day for two weeks or administered 2mg/day if pregnancy is greater than 28 weeks.21
In 2011 Trucco et al published a report of 20 cases, which were administered intravenous immunoglobulin (IVIG) associated with corticosteroids in pregnant whose fetus were accompanied with cardiomyopathy and / or secondary fibroelastosis to AV block third degree showing good results in greater survival 80%.15 The recommended (IVIG) dose is 400mg/kg/day for 5 days,15 however given the limitations of this publication, it is concluded that further studies are needed to determine the dose and duration appropriate.
On postnatal handling, Yang et al reported a series of cases in 2012 in which they analyzed a cohort of 15 patients with complete AV block, finding that 87% of infants requiring implantation of a pacemaker as definitive management.16 The timing of pacemaker implantation depends on whether the patient is symptomatic, since the ventricular rate of complete AV block is usually not sufficient to maintain cardiac output neonate.22 A lower heart rate 55 bpm to an absolute indication for use of such attachments.21
In recent years they have developed increasingly smaller and longer battery life which extends the time before a first reoperation in these patients16 pacemaker. In a case report with literature review, it is documented that the tendency is to implant fewer pacemaker unicameral, preferring bicameral (greater technical difficulty at the time of insertion) because they offer the advantage of greater sinus response and hemodynamic benefits the atrioventricular synchrony.17
In postnatal life different studies agree that mortality with early and appropriate treatment ranges from 10 to 15%; with a mortality of 5.8% when it is isolated AV block and 29-40% when it is associated with structural disease.16,17,22
Congenital third-degree AV block is an entity that although rare, has a great impact and high rate of perinatal morbidity and mortality. As its causes and diagnosis easily identifiable, it allows us to make an early diagnosis and treatment, which constitutes the fundamental pillars in addressing this disease. Although there are publications with very good results in terms of fetal and neonatal management, more studies are needed to address methodological quality and allow other options to endorse promising therapeutic regimens.
None.
The authors declare no conflicts of interest.

©2018 Poveda-Rojas, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.