eISSN: 2377-4304


Research Article Volume 14 Issue 5
1Department of Obstetrics and Gynecology, Rajavithi Hospital, Bangkok, Thailand
2College of Medicine, Rangsit University, Bangkok, Thailand
Correspondence: Assoc. Prof. Nisa Prueksaritanond, MD, Department of Obstetrics and Gynecology, Rajavithi Hospital, Bangkok, Thailand, Tel +6622062900
Received: October 14, 2023 | Published: October 25, 2023
Citation: Nisa P, Sasiwimol K, Putsarat I. Effect of acupressure on bowel function recovery after cesarean section: a randomized controlled trial. Obstet Gynecol Int J. 2023;14(5):152-158. DOI: 10.15406/ogij.2023.14.00714
Aim: This randomized controlled clinical trial aims to evaluate the effect of acupressure on recovery of bowel function after cesarean section (CS).
Methods: Forty-eight pregnant women who underwent CS at Rajavithi Hospital between December 1, 2020, and June 30, 2021, were recruited and randomly assigned into two groups: 24 participants in the study group and 24 participants in the control group. The study group received two acupressure sessions: one three hours after CS and another three hours after the initial session. The acupoint used was Zusanli (located on the stomach meridian, ST-36), and each acupressure session lasted 10 minutes. In contrast, participants in the control group followed the standard feeding protocol without receiving acupressure. The primary endpoint measured was the time to the first flatus. Secondary endpoints included the time to the first bowel sound, first defecation, the severity of nausea/vomiting, length of hospital stays, and adverse events related to acupressure, including soreness, bruising, and discomfort.
Results: Participants in the acupressure group had a significantly shorter time to the first flatus, first bowel sound, first defecation, and experienced milder nausea/vomiting compared to those in the control group. However, no statistically significant difference in the length of hospital stays and no adverse events related to acupressure were observed.
Conclusions: Acupressure, a non-invasive, feasible, and safe approach, has demonstrated its effectiveness in promoting faster recovery of bowel function in women undergoing CS. Therefore, we recommended it as an adjunct postoperative care method to reduce the incidence of postoperative ileus after CS.
Trial registration: Clinical trial registration number: NCT04620850
Keywords: acupressure, zusanli, bowel function recovery, cesarean section, postoperative ileus
CS, cesarean section; PI, postoperative ileus; BMI, body mass index; kg/m2, kilograms per meter square; SD, standard deviation
Cesarean section (CS) is a common major abdominal surgery, with global rates increasing from 6-27%.1 Despite its life-saving surgical procedure, CS is linked to maternal morbidities, including bleeding, infection, bladder, ureteric, and bowel injury, as well as postoperative ileus (PI). PI refers to a blockage of the gastrointestinal (GI) tracts characterized by abnormal GI motility and peristalsis failure.2 The pathogenesis of PI is multifactorial, including immunological, inflammation, autonomic dysfunction, agonism at gut opioid receptors by exogenous narcotics, modulation of GI hormone activity, and electrolyte imbalance.3 CS-related factors, such as opioid use, excessive manipulation, and postoperative changes in autonomic nervous systems, contribute to decreased GI peristalsis.
Following CS, a standard protocol withholds oral feeding until PI resolves, as indicated by bowel sound and the passage of flatus or stool. Prolonged PI resolution leads to breastfeeding difficulties, elevated risk of morbidities (e.g., abdominal pain, distention, oral intake difficulty, pulmonary complications, wound healing problems, prolonged immobilization, and thrombosis), longer hospital stays, and higher healthcare costs.4,5 Therefore, reducing PI duration is crucial to improve patient outcomes and lower healthcare expenses.
Clinical trials have investigated various measures to prevent or decrease PI, such as nasogastric decompression, motility agents, early postoperative refeeding regimens, physical therapy, and early immobilization.6,7 However, these approaches are not routinely used due to limited clinical efficacy. The enhanced recovery after surgery (ERAS) guideline for postoperative care in CS suggests sham postoperative feeding (chewing gum) to reduce and promote GI function recovery.8,9 Nevertheless, the quality of evidence is low, making this treatment redundant if early oral feeding is employed.
As non-pharmacological interventions, acupuncture and acupressure have been investigated as adjuvant therapies for preventing PI.10 Acupuncture and acupressure are popular alternative medicines effective for functional GI disorders after general anesthesia, CS, and gynecological laparoscopy, as supported by several reports.11–13 Although acupuncture is more familiar, it has the disadvantage of being invasive and potentially painful as it involves inserting the tips of stainless-steel needles into specific points (acupoints) and needle manipulation (e.g., lifting, twisting, and twirling).
Acupressure, a well-accepted traditional Chinese medicine (TCM), has been used for thousands of years as a simple measure of self-care and treatment for various diseases. According to TCM theory, qi-a life force flows through invisible channels in the human body, and acupoint stimulation regulates this flow.14 Acupressure aims to improve qi flow and restore the balance of yin and yang using fingers, palms, elbows, feet, or special devices to apply pressure to specific acupoints. After the surgery, GI function may be impaired due to disrupted qi flow. Acupressure is assumed to work by engaging the vagal and parasympathetic pathways, releasing acetylcholine, promoting the pituitary release of adrenal cortex hormones, and inhibiting GI inflammatory response.15 Thus, acupressure presents an intriguing approach to preventing PI in the postoperative period.
ST-36, also known as Zusanli, is located on the stomach meridian and is a commonly used acupoint to treat GI diseases. Acupressure applied to the ST-36 acupoint can increase GI motility in patients undergoing hysterectomy and colorectal surgery through parasympathetic transmission.16,17 Additionally, a systematic review and meta-analysis of randomized clinical trials (RCTs) have demonstrated that injections at the ST-36 acupoint with various agents (i.e., neostigmine, vitamin B1, and metoclopramide) might have a preventive effect on PI by reducing the time to bowel sounds recovery and first defecation.18 However, this finding should be interpreted cautiously due to the poor methodological quality of the included trials and the possibility of publication bias.
Our present study involved a well-designed RCT to confirm the efficacy of acupressure in resolving PI in patients who underwent CS. The study purposed to test the hypothesis that applying acupressure at the ST-36 or Zusanli acupoint after CS is safe, feasible and would accelerate bowel function recovery in terms of time to first passage of flatus, time to first bowel sound, time to first defecation, as well as reducing nausea, vomiting, and the length of hospital stay.
Study design and setting
This RCT study included 48 pregnant women who underwent CS at Rajavithi Hospital from 1st December 2020 to 30th June 2021. The Institutional Research Committee (IRB) of Rajavithi Hospital approved this clinical trial with the registration number 63140; 165/2563, and we prospectively registered at ClinicalTrail.gov (clinical trial registration number: NCT04620850). After approval, eligible patients were invited to participate, and the study details were explained to all participants. Patients who provided written informed consent were enrolled. Then, an independent investigator performed a block-of-four randomization using a web-based computer-generated system (www.Randomization.com). Patients were assigned randomly in a 1:1 ratio to receive either acupressure (intervention group) or usual care (control group). Randomization numbers were kept in sequentially numbered sealed-opaque envelopes. After the operation, the assigned intervention was revealed and implemented by the responsible nursing staff in the postpartum service ward. Although the nature of the intervention did not permit blinding to patients and the researcher who performed the acupressure, the patient and investigators were educated to keep the group assignment secret. The outcome measurement was objective; thus, the clinicians, outcome assessors, and statisticians were blinded to the treatment assignment throughout the study’s conduct.
Participants
Pregnant women who were candidates for CS were recruited for the study. The inclusion criteria were as follows: aged 18 to 45 years; good consciousness and well-cooperated; able to communicate in Thai; had a minimum of 6-hour fasting time prior to surgery; and were undergoing CS under general anesthesia. They were excluded if they were high-risk pregnancies (e.g., pre-eclampsia, eclampsia, multi-fetal gestation, and hypo- or hyperthyroidism), previous gastrointestinal surgery, intraabdominal adhesion, placenta previa, abruption placenta, intraoperative complication (e.g., uterine atony, placenta accrete, bladder injury, and bowel injury), required hysterectomy, had operative time exceeding 2 hours, experienced operative blood loss exceeding 1,000 milliliters and required transfusion, had postpartum hemorrhage, puerperal infection, refused our assessment, or were transferred to other units such as intensive care for more than 24 hours. Patients with lower limb defects or infections that would affect the identification of the acupoint or worsen local infection were also excluded.
Intervention
Patients in the acupressure group were instructed to undergo two acupressure sessions. The first session began three hours after CS, while the second was performed three hours after the first session. The acupoint used was Zusanli (stomach meridian, ST-36), located four fingerbreadths below the lower patella and one fingerbreadth to the anterior crest of the tibia or the anterior tibialis muscle (Figure 1).16 During the acupressure procedure, pressure at the Zusanli point for 3-5 seconds, then released for 2 seconds, and continued for 5 minutes. The depth of each press was one centimeter, and the procedure was repeated on the contralateral leg. Therefore, each session of acupressure lasted for 10 minutes. The researcher who applied acupressure was trained and approved by the traditional Chinese medicine physician at the Traditional Chinese Medicine Unit of the Hospital of Tropical Diseases. To ensure the acupoint was correctly located at the Zusanli site, the researcher asked the patient whether she felt soreness, fullness, or a heat sensation. If the patient answered "yes", the acupoint was verified, and the acupressure procedure was continued. If the patient answered "no", the location of the acupressure was readjusted until the patient responded positively feel before applying the same cycle once more. For the control group, the participants did not receive acupressure but instead received standard postoperative care as usual.
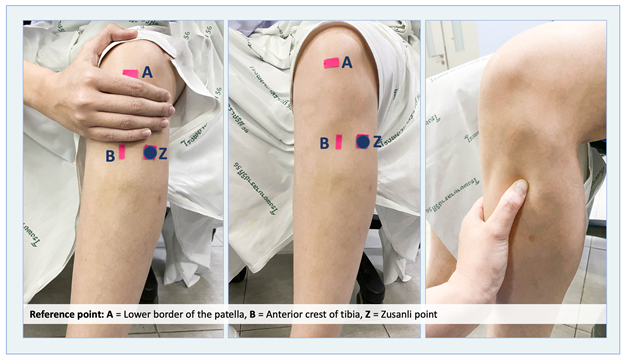
Figure 1 Zusanli (ST-36) acupoint is located four finger breadths from the lower border of the patella, lateral to the tibia).
Outcome measurement
The collected data of this study included the patient’s basic information, medical history, clinical details, and measurements of the efficacy and adverse effects of acupressure. The clinical information encompassed patient characteristics, underlying diseases, antenatal care details, CS indications, operative notes, operative time, estimated blood loss, birth weight, and other obstetric complications. The measurement of acupressure efficacy was performed by the responsible nursing staff in the postpartum service ward, who were trained and approved by the clinicians in the research team. The primary outcome was the time to the first passage of flatus after CS. The secondary outcomes included the time to the first bowel sound, time to first defecation, severity of nausea/vomiting, length of hospital, and any adverse effects of acupressure, such as soreness, bruising, and discomfort.
The first bowel sound was defined as the time when the bowel sound was first heard after the CS. The outcome assessor, who was blinded to the study allocation, evaluated the bowel sound four times daily. This assessment started 24 hours after CS and continued until the first bowel sound was noticed. The patients were instructed to notify the nurses after experiencing the first passage of flatus or defecation. Postoperative nausea/vomiting symptoms were categorized as “mild symptoms” if they resolved spontaneously without medication, “moderate symptoms” if nausea/vomiting persisted and required oral anti-emetic drugs, and “severe symptoms” if nausea/vomiting continued, necessitating anti-emetic intravenous drugs.
Study procedures
All eligible patients willing to participate in this study provided written informed consent. After that, investigators recorded the patient’s basic information, medical history, and clinical details. Before undergoing CS, all patients were required to observe a minimum fasting time of 6 hours and receive surgical preparation following the standard protocol. The numbered envelope containing randomization allocation was opened when the patient entered the operating room. Prophylactic intravenous antibiotics (cefazolin 1-2 gm or clindamycin 900 mg if penicillin allergy) were administered intraoperatively at the anesthesia induction. Consultation anesthesiologists provided general anesthesia with the same anesthetic techniques for all participants. Then, CS was performed by the resident or staff of the Department of Obstetrics and Gynecology, Rajavithi Hospital, using the same standard CS approach. After completing CS, interventions were applied to each patient based on the study arm to which they were previously assigned. However, the same standard pre-, peri-and postoperative care protocols were used for all patients.
The postoperative protocol included standard pain medication and offered anti-emetic agents such as metoclopramide. The intravenous fluid of 2,000 milliliters was administered during the first 24 hours. Regular oral paracetamol was provided, and additional opioid or nonsteroidal analgesic and anti-emetic agents were prescribed if necessary. Any additional analgesic and anti-emetic agents were recorded. The urinary catheter was removed on the first postoperative morning, and early ambulation and breastfeeding were encouraged immediately.
The postoperative feeding regimen was standardized for all patients. It began with 30-60 milliliters of water in the first 6 hours after CS. After passing flatus, a soft diet was allowed and advanced to a regular diet as tolerated. Patients in the acupressure group received their first acupressure session three hours after CS by the trained and approved researcher, followed by a second session three hours after the first. If patients experienced side effects from acupressure (e.g., pain, bruise, or discomfort), the acupressure would be discontinued, and symptom alleviation measures were implemented. The control group participants did not receive acupressure but followed the standard postoperative protocol. An outcome assessor, blinded to the study allocation, evaluated bowel sounds using a stethoscope four times daily (at 6.00 am, 12.00 am, 6.00 pm, and 12.00 pm) until the first bowel sound was detected. All patients were instructed to promptly notify the nursing ward staff when the first passage of flatus and defecation occurred. Other postoperative complications (e.g., re-operation, purpureal infection, and deep vein thrombosis) were monitored throughout the hospitalization. All patients were discharged using the standard criteria for discharge, which included stable vital signs with no fever for at least 24 hours, ambulating without assistance, tolerating a regular diet without vomiting, and no complications after CS.
Sample size calculation
The sample size calculation was based on Abadi’s study data using the formula for testing the difference in two independent means.11 In the acupressure group, the mean time to first passage of flatus after CS was 17.7 ± 6.0 hours, while in the control group, it was 25.7 ± 9.1 hours. Assuming an alpha level of 0.05 and a power of 80%, a sample size of 20 subjects was needed in each group. After adjusting for a dropout rate of 20%, a minimum of 24 subjects were required in each group.
Statistical analysis
All analyses were conducted based on the intention-to-treat principle, and all eligible patients were included in their respective groups according to the randomization. Categorical variables were compared between the acupressure and control groups using the Chi-square or Fisher’s exact test and expressed as frequencies and percentages. For continuous variables, the student’s t-test was used for normally distributed data and expressed as mean ± standard deviation. The Mann-Whitney U test was used for non-normally distributed data and expressed as median and range (minimum-maximum). The Kaplan-Meier methods were utilized to analyze the time to the first hearing of bowel sound, the time to the first passage of flatus, and the time to the first defecation. Statistical analysis was performed using the Stata software package, version 15.1 (Stata Corp, College Station, Texas, USA), and a two-sided p-value smaller than 0.05 was considered statistically significant.
During the study period, a total of 1,225 pregnant women were candidates for CS at the Department of Obstetrics and Gynecology, Rajavithi Hospital. After assessing the eligible criteria, 1,169 were excluded because of existing exclusion criteria (n=974) or refusal to participate in the study (n=8). Thus, 48 patients were included and equally randomly assigned, with 24 patients in each group (acupressure and control). One of the 48 patients who consented was excluded after being assigned to the acupressure group because she refused to continue the study. Another patient was excluded after being allocated to the control group due to postpartum hemorrhage. Among the remaining 46 patients, 23 in the acupressure group and 23 in the control group were included in the intention-to-treat analysis. The consort flow diagram and the reasons for exclusion are shown in Figure 2 .
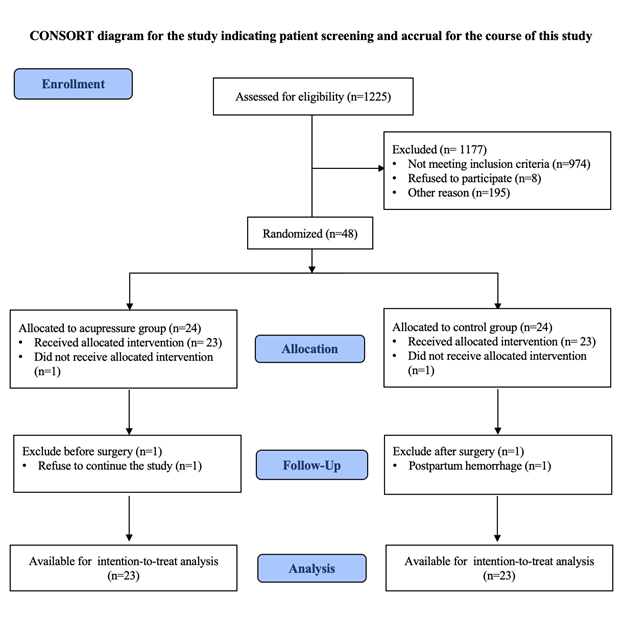
Figure 2 Participant flow diagram after randomization to either the acupressure group or the control group.
Demographic data and clinical and surgical characteristics were compared between the acupressure and the control group, and the results were presented in Table 1 and Table 2, respectively. The mean age of the patients was 29.5 ± 5.5 years, and the mean body mass index (BMI) was 29.0 ± 4.7 kg/m2. More than half of the patients were Thai, and the majority (67.4%) were nulliparous. One-quarter of the patients (23.9%) had a history of previous surgery. The mean gestational age of the patients was 38.4 ± 2.1 weeks, and the mean birth weight was 3,020.9 ± 496.2 grams. The most common indication of CS was fetal non-reassuring status (56.5%), and the most common type of skin incision was a low midline incision (93.5%). The mean operative time was 64.3 ± 17.2 minutes, and the mean operative blood loss was 432.6 ± 205.5 milliliters. However, these results showed no statistically significant difference between the two groups.
|
Characteristics |
Acupressure |
Control |
p-value |
|
(n=24) |
(n=24) |
||
|
Age (years.), mean ± SD |
29.1 ± 6.2 |
29.7 ± 4.7 |
0.681 |
|
BMI (kg/m2), mean ± SD |
29.4 ± 5.1 |
28.5 ± 4.3 |
0.528 |
|
Ethnicity, n (%) |
0.325 |
||
|
Thai |
14 (58.3) |
16 (66.7) |
|
|
Myanmar |
3 (12.5) |
3 (12.5) |
|
|
Laos |
2 (8.3) |
4 (16.7) |
|
|
Cambodia |
5 (20.8) |
1 (4.2) |
|
|
Parity, n (%) |
0.365 |
||
|
Nulliparity |
14 (58.3) |
17 (70.8) |
|
|
Multiparity |
10 (41.7) |
9 (29.2) |
|
|
Underlying disease, n (%) |
4 (16.7) |
3 (12.5) |
0.638 |
|
Overt diabetic mellitus, n (%) |
3 (12.5) |
2 (8.3) |
0.637 |
|
Previous surgery, n (%) |
6 (25) |
5 (20.8) |
0.731 |
|
Gestational age (weeks), mean ± SD |
38.3 ± 2.0 |
38.5 ± 2.0 |
0.783 |
|
Birth weight (grams), mean ± SD |
3,116.0 ± 512.1 |
2,970.7 ± 500.7 |
0.325 |
Table 1 Demographic data and clinical characteristics compared between the acupressure and the control group
BMI, Body Mass Index; kg/m2, kilograms per meter square; SD, standard deviation
|
Characteristics |
Acupressure |
Control |
p-value |
|
|
(n=23) |
(n=23) |
|
|
Indication cesarean section, n (%) |
0.712 |
||
|
Previous cesarean section |
5 (21.8) |
4 (16.7) |
|
|
Abnormal presentation |
2 (8.7) |
1 (4.2) |
|
|
Cephalopelvic disproportion |
3 (13.0) |
3 (12.5) |
|
|
Fetal non-reassuring |
12 (52.2) |
14 (58.3) |
|
|
Unfavorable cervix |
0 (0.0) |
1 (4.2) |
|
|
Failed induction |
1 (4.3) |
0 (0.0) |
|
|
Fetal macrosomia |
0 (0.0) |
1 (4.2) |
|
|
Skin incision, n (%) |
1 |
||
|
Pfannenstiel incision |
2 (8.7) |
2 (8.7) |
|
|
Low midline incision |
21 (91.3) |
21 (91.3) |
|
|
Operative time (min), mean ± SD |
65.6 ± 19.0 |
63.4 ± 15.0 |
0.688 |
|
Operative blood loss (ml.), mean ± SD |
425.0 ± 214.6 |
465.0 ± 227.3 |
0.536 |
Table 2 Surgical characteristics compared between the acupressure and the control group
min, minutes; ml, milliliters; SD, standard deviation
We found that patients who received acupressure were well-tolerated and did not experience adverse events such as pain, bruise, or discomfort. Additionally, postoperative complications, including re-operation, purpureal infection, or deep vein thrombosis, were not observed in either group.
The primary and secondary outcomes compared between the acupressure and the control group are shown in Table 3. Compared to the control group, we observed that the acupressure group had a significantly shorter interval between the end of CS and the first passage of flatus (18.6 ± 3.7 hours vs. 32.1 ± 5.5 hours; mean difference -13.5 hours (95%CI; -16.3, -10.7); p-value <0.001) and time to first hearing of bowel sound (9.9 ± 2.9 hours vs. 20.4 ± 3.0 hours, mean difference -10.5 hours (95%CI; -12.3, -8.7), p-value <0.001). Moreover, the acupressure group showed significant improvement in the time to the first defecation compared to the control group (54.1 ± 10.0 hours vs. 69.2 ± 7.9 hours; mean difference -15.0 hours (95%CI; -20.4, -9.6); p-value <0.001). The effect of acupressure compared to the control group in terms of time to first passage of flatus, time to the first hearing of bowel sound, and time to the first defecation are also shown graphically in the Kaplan-Meier curves in Figure 3. Nevertheless, the length of hospital stay did not show a statistically significant difference between the two groups: 3.4 ± 0.9 days in the acupressure group and 3.4 ± 0.4 days in the control group, with a p-value of 0.993.
|
Characteristics |
Acupressure |
Control |
p-value |
|
|
(n=23) |
(n=23) |
|
|
Primary outcome |
|||
|
Time to first passage of flatus (hours), mean±SD |
18.7±3.7 |
32.2±5.6 |
<0.001* |
|
Secondary outcome |
|||
|
Time to first bowel sound (hours), mean±SD |
9.9±2.9 |
20.5±3.1 |
<0.001* |
|
Time to first defecation (hours), mean±SD |
54.2±10.1 |
69.2±7.9 |
<0.001* |
|
The severity of nausea/vomiting, n(%) |
<0.001* |
||
|
Mild |
1 (4.4) |
13 (56.5) |
|
|
Moderate |
0 (0.0) |
0 (0.0) |
|
|
Severe |
0 (0.0) |
0 (0.0) |
|
|
Length of hospital stay (days), mean±SD |
3.4±0.9 |
3.4±0.4 |
0.993 |
Table 3 The primary and secondary outcomes compared between the acupressure and the control group
SD, standard deviation
* = significance difference (p<0.05)
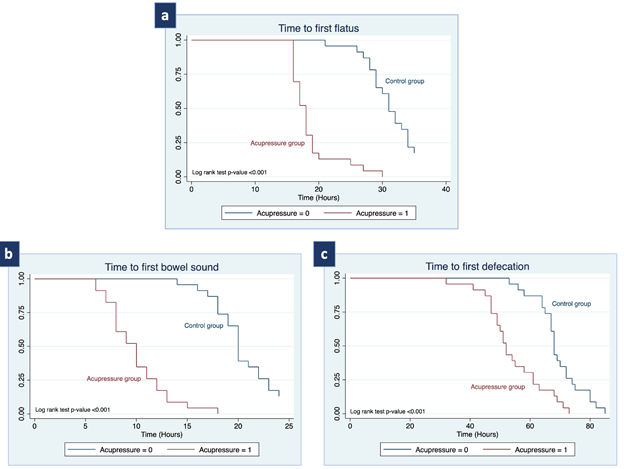
Figure 3 Kaplan-Meier represents (a) time to first passage of flatus, (b) time to first bowel sound, and (c) time to first defecation.
Mild nausea/vomiting was significantly more frequently observed in the control group, with thirteen patients (56.6%), compared to only one patient (4.4%) in the acupressure group (p-value <0.001). These patients were able to alleviate the symptoms through fasting and intravenous hydration without the need for additional medication. However, no patients experienced moderate or severe nausea/vomiting in either group. Finally, all participants were able to tolerate a regular diet before being discharged from the hospital.
PI, a common GI problem following CS operation, is influenced by predisposing factors such as bleeding, excessive manipulations of the abdominal cavity, and opioid administration. As it can affect breastfeeding and the length of hospital stay, several strategies have been suggested to reduce its occurrence, but they encounter limitations in clinical efficacy and safety concerns.4–6, 15
Acupuncture and acupressure are widely accepted as adjuncts for managing GI diseases and relieving PI.14,19 A preclinical study demonstrated that acupuncture at the ST-36 acupoint promotes PI recovery by stimulating the solitary tract neurons.20 Additionally, recent studies have shown that acupressure of the ST-36 acupoint increases GI motility in patients after hysterectomy and colorectal surgery.16,17 Although the exact mechanism is unknown, acupressure has been found to improve GI secretion, accelerate gastric emptying, and restore impaired GI motility via the cholinergic pathway.7,18,19,21 Therefore, we conducted an RCT to evaluate the effect of acupressure on bowel recovery in women who underwent CS, hypothesizing it to be beneficial.
PI is diagnosed clinically and includes symptoms such as postoperative nausea/vomiting, abdominal distention, lack of bowel sound, delayed oral feeding, delayed passage flatus, and defecation.2 A previous systematic review has highlighted the importance of time to the first passage of flatus or stool in GI motility recovery and postoperative GI function.22 As a result, most clinical trials addressing PI treatment consider this time as a primary endpoint. Our study follows this literature, defining the time to the first passage of flatus as the primary outcome to assess the effect of acupressure on bowel recovery in women after CS.
Acupressure aims to improve qi flow and regulate yin and yang balance in TCM theory. Acupoints along the meridians promote health and prevent disease.14 Acupressure at P-6 (Neiguan) acupoint significantly reduces postoperative nausea/vomiting compared to sham acupressure or usual care.23,24 However, patients' selective reporting of subjective outcomes, such as nausea/vomiting, may occur due to group allocation awareness. A systematic review and meta-analysis of RCTs revealed that ST-36 acupoint injections with various agents (i.e., neostigmine, vitamin B1, and metoclopramide) might prevent PI by accelerating bowel movement and first defecation.25 For this reason, we have selected the acupressure at ST-36 (Zusanli) acupoint, which is located on the stomach meridian and is commonly used in treating GI diseases, to investigate the effect of ST-36 acupressure on the time to the first passage of flatus, time to the first hearing of bowel sound, and time to defecation. Additionally, we have trained the outcome assessors uniformly to enhance outcome measurement validity and reliability in this study.
This study confirmed the hypothesis that administering acupressure in repeated sessions on the ST-36 acupoint reduced PI in women undergoing CS compared to the control group. Similarly, a study in Iran found that combining acupressure on the stomach meridian ST-36 (Zusanli) and large intestine meridian LI-4 (Hegu) after CS significantly reduced PI in terms of shortening the time to first passage of flatus, time to first hearing of bowel sound, and duration of postoperative bed rest.11 Our findings were consistent with Chen et al.'s study, in which acupressure at three meridian points (i.e., P-6, ST-36, and SP-6) significantly increased GI motility in women who underwent a transabdominal hysterectomy.17 However, our finding did not claim that the ST-36 acupressure offered a significant difference in the length of hospital stay. The possible reason may be that the reduction in the overall PI effect is related to combining acupoints. Stimulating P-6 alone reduced postoperative nausea/vomiting, but it was enhanced when combined with stimulation of ST-36, which adjusted GI muscular electroactivity and increased the frequency of GI contraction. Another explanation for ST-36 acupressure not showing a significant effect in decreasing the length of hospital stay may be that various factors can interfere with the length of hospital stay in women after CS, such as inability to breastfeed, insufficient breast milk, neonatal jaundice, postpartum depression, and anxiety for taking care of the newborn.
For clinical application, acupressure at the ST-36 acupoint improved PI and early recovery bowel function in women after CS without any adverse effects, such as soreness, bruising, and discomfort. Nevertheless, a cost-effective analysis should be conducted, considering health spending on PI reduction methods and trained acupressure providers before incorporating our findings into a postoperative care protocol.
Our study had several strengths, including a prospective RCT that effectively addressed the therapeutic research question. Both study groups had similar baseline characteristics, indicating a robust randomization process, and ensuring patients in both groups had a comparable prognosis. The researcher who performed acupressure on all participants was trained and licensed in Traditional Chinese Medicine, ensuring the accurate location of the ST-36 (Zusanli) acupoints. We separated the investigator and outcome assessors to prevent measurement bias. Moreover, the study was conducted in a single institution using the same standard CS operation, minimizing variation that could influence PI development.
Conversely, there were some limitations in this study. Firstly, most women included had experienced a healthy pregnancy process and successful CS operation without complications, which may limit the generalizability of the results to all women who undergo CS. Secondly, we did not have a placebo group in our study; as a result, the patients and clinicians were not blinded, and we are uncertain about the extent of its impact on the outcome measurement. While blinding was not feasible due to the nature of the intervention, the use of objective outcomes (i.e., time to first defecation and the length of hospital stay) as endpoints may have minimized the potential bias. Thirdly, the attending physician can easily manipulate certain study endpoints, such as time to first flatus and time to first bowel sound, introducing bias because of a lack of blinding for patients and clinicians. Fourthly, the duration of postoperative bed rest or the length of the hospital stay could be additional indicators of the actual effect of acupressure on bowel function recovery. Finally, the use of an opioid can influence the GI system and worsen PI. As a standard pain management protocol includes opioid administration, we did not exclude patients receiving opioids from our study. However, we recorded the dosage of additional opioids or other painkillers to evaluate whether this factor affected the study’s outcome.
ST-36 acupressure was statistically and clinically effective in reducing PI after CS. This effect was evident in the significant reduction of time to the first passage of flatus, time to the first hearing of bowel sound, and time to the first defecation. Therefore, acupressure, a simple, non-invasive, safe, and low-cost method, is a valuable strategy for promoting bowel recovery in women undergoing CS.
The authors would like to express their gratitude to the nursing staff, residents, and obstetric and gynecologic staff at the Department of Obstetrics and Gynecology, Rajavithi Hospital, for their valuable support and assistance throughout this trial.
Author contributions
NP: protocol development, data analysis, manuscript writing/editing.
SK: protocol development, data collection, manuscript writing.
PI: data analysis, manuscript writing/editing.
All authors read and approved the final version of this manuscript.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration.
This trial was supported by the Rajavithi research management fund (EC Number 63140; 165/2563).
All authors declare that they have no conflict of interests.

©2023 Nisa, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.