eISSN: 2377-4304


Review Article Volume 15 Issue 4
Diploma in Lipidology (ALALIP), Autonomous University of Baja California (UABC), Uruguay
Correspondence: Edison Edgardo Romero Galván, MD, Diploma in Lipidology (ALALIP), Autonomous University of Baja California (UABC), Private Medical Office in Tacuarembó/Uruguay
Received: August 13, 2024 | Published: August 28, 2024
Citation: Edison ER. Dyslipoproteinemias in postmenopausal women and new therapeutic targets. Obstet Gynecol Int J. 2024;15(4):169-174. DOI: 10.15406/ogij.2024.15.00755
Dyslipoproteinemias play a very prominent role in the pathogenesis of atherosclerosis in postmenopausal women; These lose the cardiovascular protection of estrogens, increasing the risk of developing atherothrombotic vascular disease (CVD).
International guidelines do not vary between the management of dyslipidemia between men and women, but these are treated less vigorously and without taking into account the postmenopausal hormonal situation.
Its management must be included in the individual risk of each woman, with the new LDL goals and above all take into account Lipoproteins rich in Triglycerides (LPRTG) that are associated with the classic modifiable risk factors, in a non-modifiable biological terrain such as are age and genetic family history; which may be associated with emerging factors such as Lipoprotein “a” (Lp a), hyperhomocysteine, elevated CRP (C-reactive protein), among other factors that are rarely taken into account.
Statins demonstrated their effectiveness in reducing LDL and even increasing HDL, reducing cardio and cerebrovascular events, but a good percentage of patients, despite the intensified doses, remain at Residual Lipid Risk, so we must resort to new therapeutic targets. like biological ones.
Keywords: dyslipidemia, risk factors, statins, biologicals
Since the first epidemiological and observational studies began in 1948 in a Framingham population recruiting 5,209 participants aged 28 to 62 years, a dense and complex database has been assembled over the last 70 years; studying the different cardiovascular risk factors (CR/CV), a concept introduced in 1961; modifiable such as smoking (1962), diabetes (1974), high blood pressure (1957), sedentary lifestyle and obesity (1967), hypercholesterolemia (1957), Triglycerides (TG) and lipoproteins (LP) (1977); that directly affect non-modifiable RFs such as age, sex and genetics (Framingham Heart Study) Figure 1, directed and organized by Dr. Gilcin F. Meadors jointly with Thomas R. Dawber and Moore; It has been one of the most important contributions in the field of cardiovascular diseases.1–3
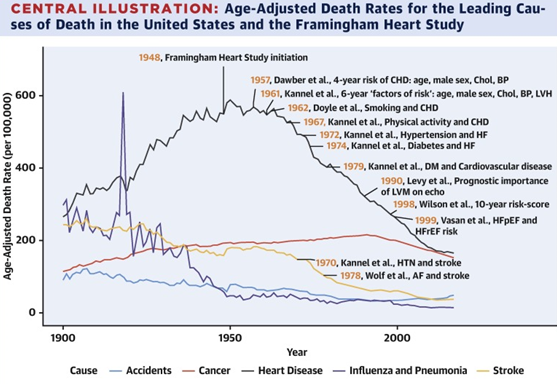
Figure 1 Framingham study started in 1948. (Taken from Anderson, C. et al. J. Am. Coll. Cardiol. 2021; 7 (21): 2680–92).
In 1967, R/CV tables began to be designed with several boxes based on age, sex, blood pressure levels, cholesterol level, and smoking, which allowed the clinician to classify his patient into a risk stratum. by Truett, Cornfield and Kannel,4 later the risk calculators appear Figure 2.

Figure 2 CV Risk Calculator that includes the different RF and also LVH (left ventricular hypertrophy, introduced by William Kannel in 1961. (Property Dr. Romero Galván, EE, 1987).
Among the traditional risk factors that the Framingham study has identified are high total cholesterol, elevated LDL cholesterol, and decreased HDL cholesterol Figure 3.
Age, despite being non-modifiable, is of great value because it relates the exposure time that an individual has with elevated LDL and in this sense the concept of multiplying the LDL level by age arises, it would give us the mgrs in years. exposed and the cumulative R. Figure 4.5
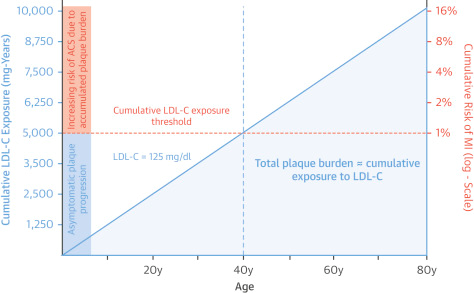
Figure 4 A 40–year–old individual with an LDL of 125 mg would be exposed to 5000 mg with a cumulative risk of 1%; from these figures the R/CV increases considerably. Multiply age x LDL = cumulative R.
Multiple prospective epidemiological studies, randomized Mendelian studies with different lipid-lowering drugs have demonstrated a decrease in the incidence of AMI with increasingly lower levels of LDL Figure 5, “without limit in its decrease”; currently 55 mgrs for very high risk patients, diabetics and hypertensives with a CV event (EAS 2019), in secondary prevention.6
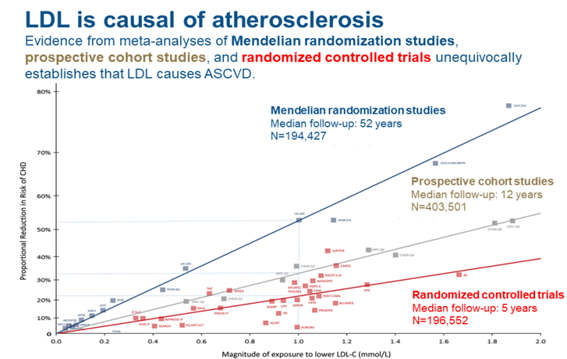
Figure 5 Decrease in R/CV with greater decrease in LDL in randomized Mendelian studies (blue), prospective studies and controlled trials (red). (From Ference BA et al. Eur. Heart J. 2017,38(32): 2459.2472).
But we should not be LDL centric since this lipoparticle (LP) is not the only one involved in the atherogenic process, atherosclerosis is due to the influx, retention and modification of atherogenic lipoproteins in the arterial intima, IDL, VLDL and their remnants, LP rich in TG (LPRTG) that easily penetrate the subendothelial space and are phagocytosed without the need for oxidation, which is why they would be more atherogenic than LDL itself. The remnants of QM and VLDL contain 20 times more cholesterol than LDL; upon passing through the endothelium, they become trapped between the proteoglycans Figure 6 without being previously oxidized. This would be the mechanism of the risk of LPRTG for atherosclerotic CVD.
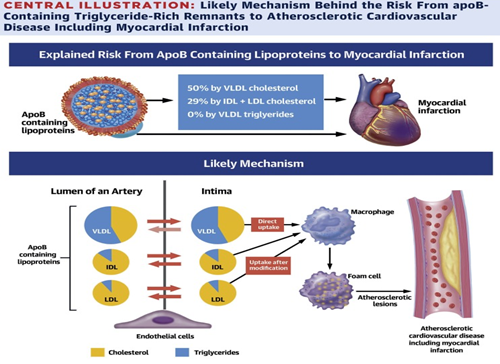
Figure 6 Passage of LP to the subendothelium, the entry of VLDL rich in TG stands out without the need for oxidation to be phagocytosed by macrophages and is an irreversible passage.6
We see in Figure 6 that the entry of VLDL into the subendothelial space, in a dysfunctional endothelium7 is irreversible, but not for IDL and LDL, which is bidirectional. These lipoparticles are captured by macrophages, transforming into foam cells, initiating the atheroma plaque.
In clinical practice, we can assess the remaining particles of VLDL, LDL (IDL), small LDL and including Lp(a) through Non-HDL Col., which few laboratories report and is calculated: Total Col. – HDL = Non-HDL Chol, which would usually be 30 mg more than the normal LDL Figure 7 and Table 1. We can also calculate by subtracting the LDL from the Non-HDL Chol (NON-HDL Chol – LDL). In RA only 32% of patients have an LDL < 70 mg; Talking only about LDL level control is already a thing of the past, we must dedicate ourselves to LPRTG, looking at all atherogenic LPs.
|
Risk (SCORE)a |
Primary target |
Secondary targetb |
|
|
LDLC mg/dL (mmol/L) |
Non-HDLC mg/dL (mmol/L) |
ApoB g/L (mg/dL) |
|
|
Very high |
<55 (1.4) and ≥ 50% reduction in LDLC |
<85 (2.2) |
<0.65 (65) |
|
High |
<70 (1.8) and ≥ 50% reduction in LDLC |
<100 (2.6) |
<0.80 (80) |
|
Moderate |
<100 (2.6) |
<130 (3.3) |
<1.00 (100) |
|
Low |
<115 (3.0) |
|
|
Table 1 Level of LDL, Non-HDL Chol and APO B in primary and secondary prevention according to risk. (From Current Cardiology Reports 2020; 22 (67):2-10).
Primary and secondary targets of preventive therapy according to cardiovascular mortality risk category assessed with the SCORE system
The importance of the knowledge and assessment of other atherogenic particles other than just LDL (Non-HDL Chol) has been demonstrated in the Copenhagen Study (CGPS) covering 13,015 patients, the group with elevated LDL, but with Non-HDL Chol. normal < 130 mg did not present an increase in mortality due to AMI; the second group with normal LDL and increased COL. NO HDL the risk of mortality was between 18 to 21% and that of AMI increased from 49 to 78%, finally a third group with normal LDL and Col. NO HDL elevated plus increased APO B the risks were 23% and of AMI 82% respectively Figure 8.
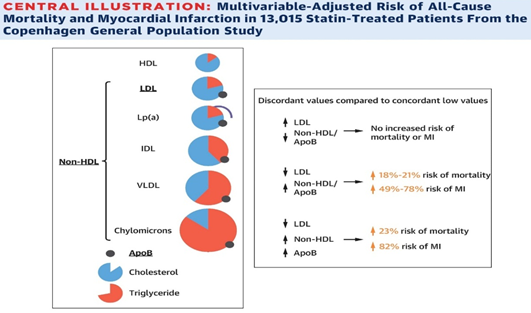
Figure 8 CGPS study. Mortality and AMI Risk according to Non–HDL Col. and APO B. (Taken from Johannesen CDL et al. J. Am. Coll. Cardiol. 2021; 77 (11): 1439–50).
Interpreting the HDL, the lipidogram only tells us a value in mgrs, as in my personal experience where the lowest HDL found has been 10 and 14 mgrs (hypoalphalipoproteinemia) as well as on the contrary the highest 139, 115, 108 mgrs in women postmenopausal, determined in the years 1989, 1990 and 2012 respectively. The mgrs interpreted in the lipid profile is something very subtle and of little value with the reality of the reverse transport of cholesterol, because it will depend on the amount of APO A 1 that said particle contains; According to the APO A as well as other APOLP we have more than 12 different HDL, we should know how each of these particles work, regardless of their value but also their functionality, where the concept of “dysfunctional HDL” arises. The patients who were treated in 1989, 1990 with HDL more than 100 mg, we thought at the time that they were very protected from AMI, today we know that it is a false premise, due to ignorance of the capacity of reverse cholesterol transport. The determination of APOA1 and APOB100 would be more faithful, as well as their ratio, being less than 1.3, it is correlated as a better discriminator of atherogenesis than Chol/HDL, marking high risk, as already observed by Jean Charles Fruchart in France (1982). The greater the APOA1 (80 to 220 mgrs), the better the protection, it has more capacity for recycling Col than other APOA. We have begun to determine this ratio in Uruguay in September 1988, in 600 patients, presented at the Second SOLAT Congress (Caracas, 1993).8
If we have LDL more than 100 mg and an HDL more than 45 mg, we do not know how much we are protected from CVD because we do not know how much that HDL carries. On the contrary, it has been shown that the higher the HDL value, the protective benefit is lost. We must change the paradigm based on the study by Chang Liu (2022)9 in 14,478 participants from the UK Biobank (2006 to 2022) and 5,467 from the CV Emory USA Biobank (2003 to 2022), with an average age of 62 years. all with a personal history of CVD and the group that had HDL more than 80 mg, 71% had a higher risk of CV death than the group that had HDL between 40 and 60 mg, a “U” curve, Figure 9. Too low or excessively high concentrations of HDL are harmful, only moderate concentrations are cardioprotective between 40 and 60 mg; Less than 30 mgrs and greater than 70 mgrs would cease to be, especially in patients with pre-existing CVD.
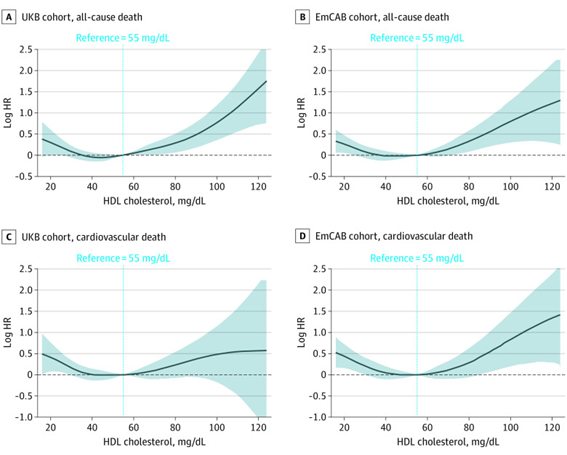
Figure 9 Cardiovascular deaths increase when HDL is less than 40 mg and when it is greater than 60 mg, “C” Biobanco UK and “D” Emory USA.
When interpreting a lipid profile, not only look at the LDL level, depending on whether it is primary or secondary prevention, less than 100 mgrs and less than 70 respectively; In those patients with very RA we must reach less than 55 mgrs according to EAS 2019 guidelines, we should stop being centric LDL and take into account the years of vascular exposure to these high values. Assess more the other lipoparticles such as LPRTG, know the level of Non-HDL Chol, APOB and APO A1, do not calm down with an HDL greater than 60 mg because it is non-protective given its dysfunctionality and according to its content of the different APO A , the most efficient HDL in the reverse transport of cholesterol are those with the highest content of APOA1, it is important to know its level, as well as the APOA1/APOB100 ratio, as a better risk discriminator than the Chol/HDL ratio itself, as already l we mentioned.
In the face of an LDL of 190 mg or more, with normal Triglycerides (<150 mg) and with a family history of cardiovascular events, a genetic study is indicated to confirm Familial Hypercholesterolemia (FH) or Muller-Harbitz Disease,10 being the The heterozygous is more common 1/300 to 500 people, the homozygous is 1/million inhabitants. Among the different mutations, the LDL receptor predominates by 90%; 2900 mutations have been described that are located on the short arm of chromosome 19 (19p13.2), increasing cardiovascular risk by 22 times. It is followed by APOB100 with a frequency of 5 to 10%; 32 mutations of its gene have been described on chromosome 2 (2p23-24). The first two cases in Uruguay were found in 2013. Even less frequent is the mutation of PCSK9 (protein Convertase Subtilisin/kexin type 9, 1%, with 23 mutations of its gene on chromosome 1 (1p32.3); it is Its identification is important due to its therapeutic management with specific Monoclonal Antibodies such as Alirocumab and Evolocumab, blocking it directly or blocking its synthesis through Inclisiran (siRNA: small RNA interference). Finally, the LDLRAP1 mutation is very rare, only 17 mutations have been described of the gene located at 1p36.11, is a cytosolic adapter protein of the LDL receptor that binds to its cytoplasmic portion to mediate its internalization to the hepatocyte.11,12 In my personal statistics, the first patient studied was in June 2011 with a Norwegian mutation, in exon 9 a Cytosine changes to Guanine, producing an amino acid change from Leucine to Valine at position 380 of the LDL receptor, which explained its value of 309 mgrs and with a history of family heart attack. Her two daughters, ages 35 and 38, also have the same mutation. Currently there are 80 patients studied with an LDL receptor mutation and only one with a PCSK9 mutation.
Therapeutic behaviour: The therapeutic options for these patients are multiple, education in changing lifestyles, with a healthy diet and physical exercises is the first behavior to take into account, despite having only a 25% impact on dyslipidemia; We have pharmacological therapy with Statins, preferably water-soluble ones (Rosuvastatin or Pitavastatin) to avoid their interactions with other drugs such as fat-soluble ones (Atorvastatin, Simvastatin) which, because they are metabolized through Cytochrome p 450 isoenzyme 3 A 4, have a greater interaction with others. drugs. The doses are escalated until reaching maximum doses, “dose intensification” (80 mg of Atorvastatin or 40 mg of Rosuvastatin) and if the goals are not reached, Ezetimibe is associated, which inhibits the Niemann-Pick C1 like protein at a level. of the enterocyte, reducing cholesterol absorption by 90% and lowering LDL by 20%. New therapeutic targets have been described with the advent of Biologics, with decreases in LDL levels never before obtained, more than 50% as in the ODYSSEY Study with Alirocumab to block the PCSK9 protein Figure 10.
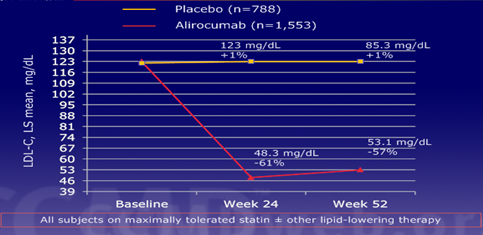
Figure 10 LDL decrease by 61% at 24 weeks in 1,553 patients versus placebo treated with Alirocumab. (Taken from Robinson JG et al. Presented at European Society of Cardiology Congress 2014. Spain).
The FOURIER Study with Evolocumab with 27,500 patients with a history of heart attacks and stroke with LDL greater than 70 mg and non-HDL cholesterol more than 100 mg, the decrease was 59% with 140 mg subcutaneously per week or 420 mg per month Figure 11.13
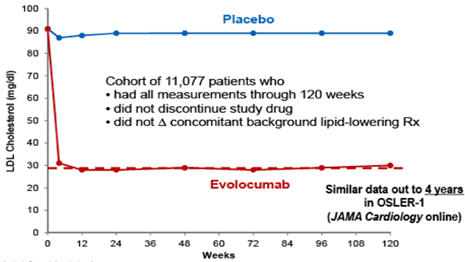
Figure 11 LDL response with weekly Evolocumab vs Placebo. (EAS, 2017. Prague; taken from Sabatine MS et al. NEJM 2017; 376: 1713–22.
These results have been reflected in a 15% decrease in MACE, 27% fewer infarctions, 21% fewer strokes and 22% fewer coronary revascularizations.14
Both Monoclonal Antibodies, Alirocumab and Evolocumab, have been compared, the latter being more effective in lowering LDL Figure 12.
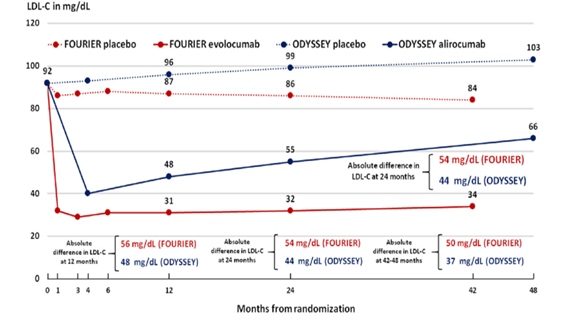
Figure 12 Comparison of results on LDL level with Alirocumab in the ODYSSEY Study and with Evolocumab in the FOURIER (Taken from Remo HM Furtado et Giuliano RP Cardiol. Ther. 2020, 9: 59–73).
By blocking the overexpression of PCSK9, it does not allow the LDL Receptor to be degraded and it can be recycled in the hepatocyte membrane to capture LDL and internalize them; Not only can we block this regulatory protein, we would also act on its synthesis directly at the ribosome level with Inclisiran, which iss an siRNA directed against mRNA encoding PCSK9, conjugated with trientenary carbohydrates N acetyl galactosamine that interact with Hepatocyte Asialoglycoprotein Receptor (ASGPR) Figure 13.
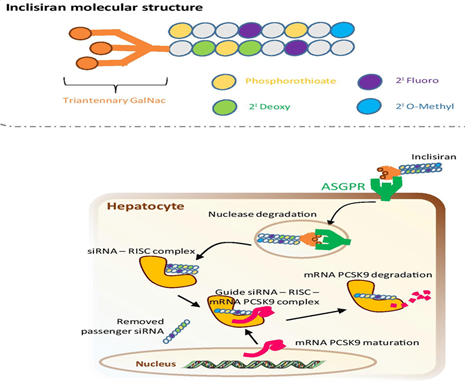
Figure 13 Inclisiran bound to ASGPR to enter the hepatocyte and form an siRNA–RISC complex to cleave the target RNA.
Once entered into the hepatocyte by endocytosis through its binding to ASGPR, it is trapped in endosomes, deposited intracellularly, and slowly released, allowing dosage every 6 months.
The RISC complex (RNA Induced Silencing Complex) is from the family of endonucleases. They are Ribonucleoproteins called Argonauts and TBRP that cut the strand of the target mRNA or siRNA (silencing or small interference) formed by 20 to 25 nucleotides that interfere with the expression of the gene. specific, in this case PCSK9, reducing it, silencing it and thus blocking its synthesis by inhibiting transcription, leading to an increase in recycling of the LDL receptor, frankly lowering its levels in plasma.15
This mechanism leads to a decrease in PCSK9 of 78.23%, which is reflected in a decrease in LDL of 52.3%; results of ORION Studies 10 enrolling 1561 patients and 11 with 1617 patients Figure 14 and Figure 15.
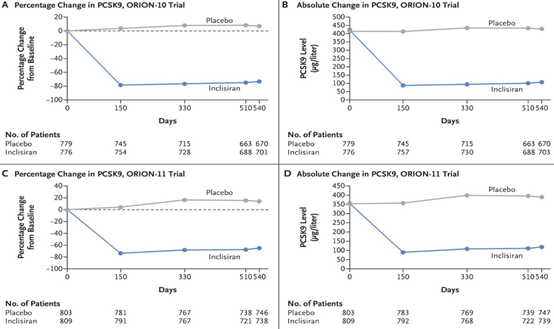
Figure 14 Decrease in PCSK9 due to the effect of Inclisiran at 150 days and remaining the same at 540 days vs placebo, ORION Trial 10 and 11.
These clear decreases have a direct impact on the reduction of cardiovascular events, MACE by 30%, higher than those obtained if we only block PCSK9 with Alirocumab or Evolocumab. Figure 16.
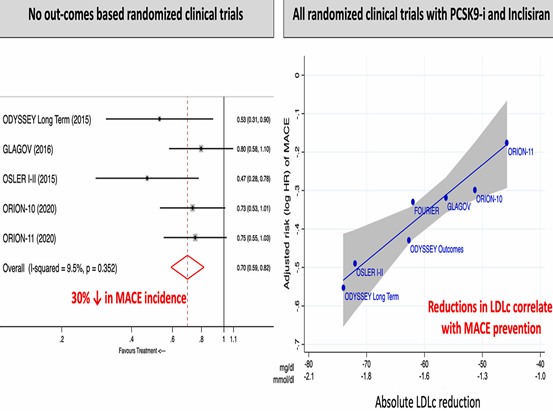
Figure 16 ORION 10–11 meta–analysis plus trials inhibiting PCSK9 with Alirocumab (n: 11,015) and Evolocumab (n: 17,244) (16).
The new therapeutic targets with these biological drugs have made it possible to reduce the residual lipid and cardiovascular risks, which despite aggressive management with dose intensification of Statins were at 70% Figure 17 as reported by the 4S Study with Simvastatin.16,17
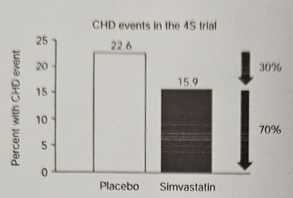
Figure 17 Residual cardio vascular risk despite treatment with Statin, Simvastatin in Study 4 S. (Taken from Ferdinand KC et al.).
Patients who have reached LDL: 70 mg but persist with high TG and HDL less than 35 mg, their CV risks still reach 40%.18,19 These residual risks would be explained in part by not increasing HDL to desirable and functional levels, TG greater than 200 mg, due to an increase in Chol. No HDL above 100 to 130 mg (in primary or secondary prevention), due to an increase in APOB100 (> 100 mg) with a decrease in APOA1 (< 135 mg), due to small and dense LDL that cross the dysfunctional endothelium more easily, due to an increase in CETP and a decrease in LPL activity.
Dose intensification with Statins is proposed following a therapeutic ladder Figure 18, moderate intensification if the risk is 30%, high doses (80 mg of Atorvastatin) with a risk of 50%, then associating Ezetimibe until reaching statin blockers. PCSK9 and even these associated with Statins plus Ezetimibe.20
Post menopause is a negative event to alter the lipid profile, which is a proven cardiovascular risk factor, with polygenic components, often associated with other modifiable risk factors such as obesity, hypertension and type II diabetes that occur in these hormonal stages of women. We highlight the time of exposure to high LDL and take into account the other lipo particles rich in Triglycerides. In patients with a family history and LDL greater than 190 mg, genetic studies should be performed due to probable mutations in the particle receptor gene. We have very effective drugs such as Statins, escalating doses, associating with Ezetimibe and if we do not reach the goals, we resort to innovative drugs that alter the central dogma of molecular biology such as Evolocumab, Alirocumab and Inclisirán in order to reduce vascular events and associate them with Statins plus Ezetimibe, we will be able to reduce the residual lipid risk by reaching the goals established by consensus, without ceasing to take into account changes in lifestyles.
To the clinical analysis laboratory Dr. Graciela Vázquez of Tacuarembó for carrying out the same on my patients, as well as the Genetic studies.
For being part of the ALALIP and the Pan-American College of Endothelium, having completed the Diploma in Lipidology (2022) and being part of the National Lipid Network for Uruguay for the EAS (2024).
None.
I have no conflicts of interest.

©2024 Edison. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.