eISSN: 2377-4304


Case Report Volume 7 Issue 2
1Department of Obstetrics and Gynecology, Southern California Kaiser Permanente, USA
2St. George's University, West Indies
3Pace University, School of Nursing, USA
4Memorial Sloan Kettering Cancer Center, USA
5Department of Obstetrics and Gynecology, Lincoln Medical and Mental Health Center, USA
6Department of Pathology, Southern California Kaiser Permanente, USA
Correspondence: Cassandra E Henderson, Pace University, School of Nursing, 1 Pace Plaza, New York, NY, 10038, USA
Received: April 07, 2017 | Published: May 26, 2017
Citation: Rezai S, Hughes A, Ligorski J, et al. Disseminated Peritoneal Leiomyomatosis (DPL); a case report and review of literature. Obstet Gynecol Int J. 2017;7(2):220-223. DOI: 10.15406/ogij.2017.07.00240
Background:Disseminated peritoneal leiomyomatosis (DPL) also known as leiomyomatosis peritonealis disseminate (LPD) is a rare condition described as multiple smooth muscle tumors disseminated throughout the peritoneum, omentum and pelvic structures. Since first being described in 1952, less than 150 cases have been reported in English literature. However, the incidence of DPL may be under estimation as many cases are asymptomatic. The lesions occur most commonly in women of child bearing age and may have an association with oral contraceptive therapy (OCP) or hormone replacement therapy (HRT). These diffuse tumors are usually incidentally found during surgery but women may present with abdominal pain prompting investigation. This condition is rarely reported; therefore we describe a case of DPL in a 35 year old woman incidentally found during cesarean delivery.
Case:34 year old female Gravida 2, Para 0010 Hispanic female, with history of asthma and no past surgical history, with uncomplicated antenatal course, was admitted for induction of labor for postdate gestation at 41 3/7 weeks, underwent a primary cesarean delivery due to failed induction of labor at 41 5/7 weeks. A large exophytic mass was incidentally noted after delivery of fetus and exteriorizing the uterus. Further biopsy of the mass was consistent with diagnosis of DPL.
Conclusion: DPL may be found incidentally during surgery and are often diffuse throughout the abdomen, involving multiple structures; therefore an intra operative consultation may be required. The risk for recurrence, impaired quality of life, obstetric complications, and possible malignant transformation underlines the importance of accurate diagnosis facilitating patient specific treatment.
Keywords: Disseminated fibro sing deciduosis, Disseminated peritoneal leiomyomatosis, Leiomyomatosis peritonealis disseminate, Leiomyomatosis peritonealis disseminate.
DPL, Disseminated Peritoneal Leiomyomatosis; LPD, Leiomyomatosis Peritonealis Disseminate; OCP, Oral Contraceptive Therapy; HRT, Hormone Replacement Therapy
Disseminated peritoneal leiomyomatosis (DPL) also known as leiomyomatosis peritonealis disseminate (LPD) is a rare condition described as multiple smooth muscle tumors disseminated throughout the peritoneum, omentum and pelvic structures.1,2 It was designated as LPD by Taubert.3,4 Since first being described in 1952 by Wilson & Peale3,5 less than 150 cases have been reported in English literature.6,7 However, the incidence of DPL may be underestimated, as most cases are asymptomatic.6,7 The lesions occur most commonly in women of child-bearing age and may have an association with oral contraceptive therapy (OCP)8 or hormone replacement therapy (HRT),6-10 pregnancy11-14 and endometriosis.15 Etiology is unknown. However, some have hypothesized that DPL occurs due to hormonal stimulation of smooth muscle cells.2-16 These diffuse tumors are usually incidentally found during surgery.10-30 Including surgical treatment of endometriosis, as there have been reported cases of DPL associated with endometriosis.6-17 This condition is rarely reported; therefore we describe a case of DPL in a 35-year-old woman incidentally found during cesarean section.
A 34 year old Hispanic female, Gravida 2, Para 0010, with history of asthma, no past surgical history and uncomplicated antenatal course was initially admitted for postdate (41 weeks 3 days) induction of labor. After failed induction, the patient was transferred to the operating room for primary cesarean delivery at 41 weeks and 5 days. At the time of cesarean section, after delivery of the fetus and exteriorization of the uterus, omental and peritoneal masses were noted arising from the posterior wall of the uterus occupying the posterior pelvis. The exophytic mass was purple, rubbery, poly poid and appeared to extend from the uterus over the bowel, peritoneum and abdominal viscera with “finger” like projections. Due to the diffuse nature of the mass, the exact size of the mass could no be determined Bilateral filmy adhesions from the mass to both adnexa were noted, though there were more on the right than left side. The fallopian tubes and ovaries appeared grossly normal bilaterally. The uterus appeared grossly normal with the exception of this exophytic mass. The peritoneal surfaces appeared smooth and without excrescences. An intra operative general surgery consult was requested and part of the mass was excised for biopsy. Peritoneal washings were obtained. Postoperatively, the patient had an uneventful recovery period and hospital course and was discharged home on postoperative day two.
It is remarkable that none of the obstetric ultrasounds during the pregnancy revealed existence of such a mass and that the patient was asymptomatic. The patient did not have any other previous abdominal imaging done prior to pregnancy.
Pathology of the mass showed soft tissue with smooth muscle proliferation, fibrotic and decidual reaction (Figure 1 & 3). There was no evidence of malignancy. Sections show a low grade spindle cell proliferation, fascicular in appearance, forming small and large nodules. The spindle cells show no cytological atypia and no mitoses were identified. The nodules are situated in a fibrous and vascular background with interspersed collections of decasualized cells. The spindle cells are positive for smooth muscle action (SMA), and are negative for Melan-A and HMB-45. The findings, combined with the clinical presentation, are consistent with disseminated peritoneal leiomyomatosis (DPL). A leiomyoma arising from the serosal aspect of the uterus is also a consideration, though less likely (Figure 1 & 2).

Figure 1 Hematoxylin and Eosin Stain (H&E stain or HE stain).
1A: Smooth muscle arranged like leiomyomas = Leiomyomatosis (Purple Circles)
1B: Stromal cells resembling decidua (Light Purple),br>Vessels (red circles)
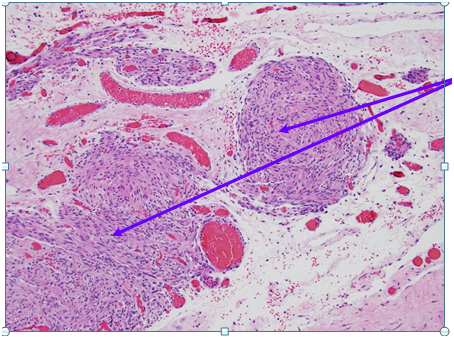
Figure 2 Higher Magnification of Box 1A from Figure 1: Smooth muscle arranged like leiomyomas / Nodules of smooth muscle bundles proliferation which are bland looking and lack mitoses = Leiomyomatosis (Purple Circles) Hematoxylin and Eosin Stain (H&E stain or HE stain). 1A: Sections show a low grade spindle cell proliferation, fascicular in appearance, forming small and large nodules. The spindle cells show no cytological atypia, and no mitoses are identified. The nodules are situated in a fibrous and vascular background with interspersed collections of decidualized cells. The spindle cells are positive for SMA, and are negative for Melan-A and HMB-45. The findings, combined with the clinical presentation, are compatible with disseminated peritoneal leiomyomatosis. A leiomyoma arising from the serosal aspect of the uterus is also a consideration, though considered less likely.
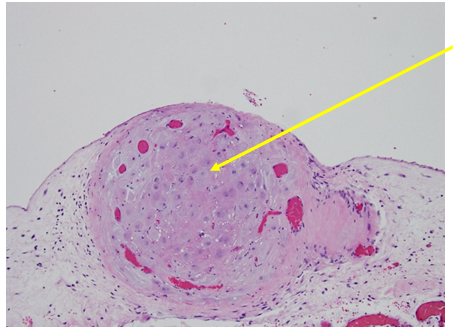
Figure 3 Higher Magnification of Box 1B from Figure 1: Stromal cells resembling decidua, Hematoxylin and Eosin Stain (H&E stain or HE stain).
A follow up appointment with gynecologic oncology was recommended for twelve weeks postpartum once the physiologic changes of pregnancy and postpartum resolved.
DPL is a rare condition of smooth muscle tumors disseminated throughout the abdomen and pelvis often occurring in women of childbearing age,7 although cases of DPL in male with malignant transformation in male have been described as well.18As discussed previously, an increase in hormone levels and the response of susceptible smooth muscle cells have been suggested as a cause of these tumors.6 However, several cases have been reported of iatrogenic dissemination after morselization either post robotic or laparoscopic surgery.7-21 As these tumors are normally discovered as an incidental finding, histology and peritoneal washing play an important role in diagnosis and ruling out malignancy. DPL tumors are traditionally considered benign but several cases of malignant transformation have been reported, many occurring after surgical treatment.18-26 In this case, a portion of the tumor was resected and sent for pathological analysis prior to follow up and further management. As stated above in the case presentation, the histo pathological findings did not suggest malignancy, but rather the smooth muscle bundles which were SMA positive suggested DPL.27 The potential for malignant transformation and the possible iatrogenic etiology makes close follow up important in patients with DPL. As with many tumors, treatment can involve hormonal therapy and surgical resection. Wu2 in 2015 found a case of estrogen and progesterone receptor sensitive DPL, supporting the etiological theory of hormonally responsive smooth muscle tumors. Conversely, a decrease in estrogen and progesterone has shown to decrease tumor size, further supporting Wu’s findings.2 Therefore in women who do not wish to become pregnant a total abdominal hysterectomy, salpingo-oophorectomy, omentectomy and debulking surgery is the best option.6,7 However, non-surgical options involve limiting hormonal exposure which have been shown to decrease tumor size.2 These include avoiding oral contraceptives (OCP) and hormone replacement therapy (HRT),10 administering gonadotropin releasing hormone agonist aromatase inhibitor therapy28,29 and avoiding pregnancy.6-28 Without proper treatment, DPL can cause complications such as pain, bleeding, chronic constipation and infertility.17-30 Therefore management of DPL should be patient centered in order to achieve the specific treatment goals for each patient depending on their future plans for pregnancy.
DPL is a rare condition described as multiple smooth muscle tumors disseminated throughout the peritoneum, omentum and pelvic structures, most commonly occurring in women of childbearing age1,2 and sometimes incidentally diagnosed during the pregnancy11-14 or at the time of cesarean delivery.12 Cases of disseminated peritoneal leiomyomatosis after robotic, laparoscopic and open+ power morcellation have been reported (17-32). These tumors may be found incidentally during surgery and are often diffuse throughout the abdomen, involving multiple structures; therefore an intra operative consultation may be required. Cases of recurrence with continued exposure to hormones such as during pregnancy, OCP and hormone replacement therapy have been reported. The risk for recurrence,33 metastasis,34 colonic obstruction,35 impaired quality of life, obstetric complications, and possible malignant transformation22-26 underlines the importance of accurate diagnosis, facilitating patient specific treatment.
The authors would like to thank Ms. Judith Wilkinson, Medical Librarian at Lincoln Medical and Mental Health Center Science Library for providing the reference articles.
None.

©2017 Rezai, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.