eISSN: 2377-4304


Research Article Volume 10 Issue 6
1Evangelical College of Paraná, Curitiba, Paraná, Brazil
2Pontificial Catholic University of Paraná, Brazil
Correspondence: Marcos Paulo R Sanches, Evangelical College of Paraná, Curitiba, Paraná, Brazil
Received: October 25, 2019 | Published: November 19, 2019
Citation: Sanches MPR. Correlation between histological type of epidermoid carcinoma in the head and neck and the presence of HPV assessed by P16 imuno-excession. Obstet Gynecol Int J. 2019;10(6):401-406. DOI: 10.15406/ogij.2019.10.00474
Introduction: Head and Neck Squamous Cell Carcinoma (HNSCC) is a malignant disease entity with a high prevalence in the world population. Among its major risk factors, there is a persistent infection with Human Papilloma Virus (HPV), which has been related to a better prognosis in patients. HPV infection results in an immunoreactivity of p16 protein that has been used as a marker of the oncogenic lineage by this etiologic agent. The lack of national case series relating the HNSCC with HPV and p16 encouraged us to develop this research.
Objective: To analyze epidemiological aspects of patients affected by HNSCC (age, sex and location of the lesion), and relate them to the prevalence of HPV infection. To evaluate the presence of virus stigmas in the samples (koilocytes). To correlate histological types and differentiation of HNSCC positive for p16.
Methods: We have analyzed 51 cases of HNSCC diagnosed between January 2005 and December 2015, seeking epidemiological profile of patients, and being the samples subjected to histopathology and immunohistochemistry for p16.
Results: Of the 51 cases evaluated, 42 were males and 9 females, mean age of 61 years. There was a higher percentage of tumors affecting the larynx (43%), with higher prevalence of keratinized cancers on non keratinized. Koilocytosis was observed in 56.9% of cases, and immunostaining for p16 was 49.02%, predominantly in tumors not keratinized (p=0.03532).
Conclusion: The present study has demonstrated that the infection prevalence of HPV in HNSCC, through the immunostaining with p16, was present in 49.02% of cases. Toward the epidemiological profile, carcinomas were more common in male individuals with middle ages of 61 years and in the larynx as more often topography. Koilocytosis was found in 29 cases, corresponding to 56.86% of our sample. The immunoreactivity of p16 protein predominated in non-keratinized tumors.
Keywords: head and neck carcinoma, human papilloma virus, p16
HNSCC, head and neck squamous cell carcinoma; HPV, human papilloma virus; SCC, squamous cell carcinoma; INCA, National Cancer Institute José Alencar Gomes da Silva; NQ, non-keratinized; PCR, polymerase chain reaction; IHQ, immune-histochemistry; RBP, retinoblastoma protein; TMAs, tissue micro arrays
Head and Neck Cancer is a broad term used to describe a diverse group of malignancies, originated in the upper aero-digestive tract, including the oral and tongue cavity, pharynx, larynx, salivary glands, ear, nasal cavity and paranasal sinuses. Among its main representatives: the squamous cell carcinoma (SCC), described as an aggressive epithelial malignant disease that accounts for 95% of tumors in these locations, being more common in the mouth.1 This specific species of tumor is so significant that, in 2012, it was estimated that the SCC of the tongue, oral cavity, pharynx and larynx together totaled in 686,300 new cases and 375,700 cancer deaths worldwide.2 And, according to data from the National Cancer Institute José Alencar Gomes da Silva (INCA), in 2016 there were 15,490 cases of oral SCC in Brazil, 11,140 in men and 4,350 affecting women.
Regarding their classification, head and neck SCC (SCCPS) are subdivided into Non-Keratinized (NQ), Keratinized (K) and Hybrid (H).3,4 The non-keratinized form is the one with the best prognosis and, when found in its basaloid differentiation, the classic form of carcinoma presentation.3,4
HPV was defined as a risk factor for cervical cancer in the 1970s; being responsible for more than 90% of malignant neoplasm of this organ nowadays. However, it has still been separately associated with oral and oropharyngeal squamous cell carcinoma, especially regarded to palatine tonsil involvement.5 The incidence of HPV-positive CECCP worldwide ranges from 20 to 80%, with the majority of incidence occurring in patients aged 40 to 55 years, without risk factors and linked to persistent high-risk HPV infection.6
This new type of HPV-positive tumor develops mainly in the palatine tonsils and tongue and manifests different epidemiological, molecular and clinical characteristics than classic CECCP. Among the main risk factors there is prognosis for white male individuals whose number of partners is high and engage in oral sex, and bearers of genital warts.7 In addition, it was discovered that the natural history of development of this disease shows a better prognosis compared to cases of patients with HPV-negative tumors.8
The incidence of HPV-infected malignancies has been increasing, as shown by Attner et al.,9 providing us with evidence to support the considerable increase in HPV-positive tongue-based cancers from 1998 to 2007. Therefore, Virus detection methods in head and neck carcinomas have risen in numbers because, aside from instructing the nature of tumors, this identification also helps to assess patient prognosis.10
HPV is a double-stranded DNA virus which family spans over 170 subtypes.11 It presents tropism by skin and mucosal epithelial cells, and its replication occurs in cell nuclei (Martinez, Avila, Caballero, 2012). After infectation, the main cytopathic effect of the virus is the development of koilocytosis, which is defined by increased concentration of koilocytes, a specific cell type and pathognomonic to HPV infection. (Silva, 2010). This element is a superficial cell with cytoplasmic cavitation and nuclear atypia.12
HPV DNA molecular detection is the gold standard method for virus identification and can be performed by hybrid capture and polymerase chain reaction (PCR), FISH, or immune-histochemistry (IHQ) for protein p16.2,6,10,11 This latter method is emphasized in the present study because, while p16 biomarkeration in HPV negative CECCP is extremely rare, the positivity of this same protein in virus-infected lesions has been frequent. In addition, the positivity of p16 biomarker in head and neck squamous cell carcinomas has been proposed as a reliable and reproducible prognostic marker.13 Cyclins are proteins related to the promotion of cell growth and are expressed in the middle and end of the G1 phase. Over expression results in increased cell proliferation, and in carcinomas, increased expression is related to aggressiveness, infiltration and metastasis (Suresh, 2015).
In regard to molecular biology, high-risk carcinogenic HPV continuously and irregularly expresses oncoproteins E6 and E7. This anomalous production results in cell immortalization and genomic instability, which events may lead to the progression of cancer-infected tissue.14 The significant increase in E7 oncoprotein by functionally inactivating the retinoblastoma protein (RBP) triggers the increase of p16 expression, which has a diagnostic relevance because it is detected by the immuno-histochemistry technique.15 The increase in E6, in turn, has the role of complementing the action of E7 by containing the pro-apoptotic cellular response by promoting unscheduled DNA replication and encouraging cell proliferation.16
It is known that there are national studies that correlate histological type of head and neck neoplasms with HPV, and few studies correlate their viral stigmas (koilocytes) with p16 biomarkeration. Furthermore, it is a known fact that HPV positivity is considered to be an independent and strong prognostic factor for CPEC. Therefore, studies such as the present study are necessary to confirm the prevalence of HPV-positive cases of CPEC, and to prove its relationship with p16 protein labeling.
The main goal
To evaluate the prevalence of HPV infection in cases of Head and Neck Squamous Cell Carcinoma, through P16 positive.
Specific goals
The research was performed in a cross-sectional and retrospective manner in the electronic archives of the Curitiba Pathology Center (Nossa Senhora das Gracias Hospital, Curitiba, Paraná) and the Hospital Universitário Evangélico from Curitiba, searching for all cases with anatomopathological diagnosis of head and neck SCC, having occurred from January 2005 to December 2015. The project was approved by the Research Ethics Committee of the Evangelical Society of Paraná - CEP/SEB, under opinion number 1.453.251.
From the selected patients, data related to gender, age and topography of the lesion were compiled from the medical records. From the archives, paraffin blocks and histopathological slides stained by the Hematoxylin and Eosin (HE) technique were obtained, both containing samples previously diagnosed with CECCP. Exclusion criteria were incomplete medical records, cases in which the slides and blocks selected by the electronic medical record were not found, as well as those that, after mounting the TMA, showed no neoplasia.
The histopathological slides were revised under multi-head microscopes in order to classify the histological type of the lesion, its differentiation, and also to verify the presence or absence of characteristic HPV (koilocyte) histological stigmas. In HE, CECCP were cataloged as "Keratinized", when they presented corneal pearl and individual keratinization; "Non-Keratinized" when they did not show signs of keratin production; and "Mixed", when the areas that had both characteristics were present. Identification of koilocytes was characterized by cells of the intermediate or superficial stratum of the epithelium, with well-marked cytoplasm clear halo and nuclear atypia (Figure 1).
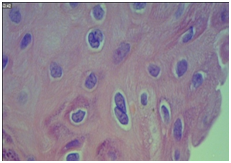
Figure 1 Coilocyte in squamous neoplasia.
Note: Photomicrography demonstrating koilocyte (arrow) in squamous neoplasia. Hematoxylin-Eosin 400x magnification.
After selecting the histopathological blocks and slides containing the carcinoma samples, the cases were reviewed in order to select the sample areas for the making of the multi-sample tissue blocks (or TMAs=tissuemicroarrays). Following the preparation of the multi-sample blocks (TMAs), these were used to prepare the multi-sample slides, in which the p16 biomarker was tested by immune-histochemistry.
Deriving from the histological slides referring to the study cases, the representative sample regions of the lesion were selected and duly marked with a rear projection pen. Through the mirror system, the marked blade is used to locate the same region in the donor block. The technique of multisampling blocks or receptors (TMAs) consists of drilling the donor block in the sample area previously marked with the consequent obtention of a tissue cylinder inside the needle and; thereafter, implantation of this tissue into the receptor block. Each sample fragment taken from the donor block was placed in the recipient block according to the “Cartesian plane” map - the columns are identified with letters and the rows with numbers (Figure 2).
The histological slides from the artisanal TMA blocks were submitted to the immune-histochemistry process, in which the study of p16 protein (FLEX TRS HIGH, Dako ®) was established. This latter process was divided into two distinct phases: first, the application of the standard immune-histochemistry technique to the material through the AutostainerLink 48 automatic staining machine, Dako®. Subsequently, reading of the area with tissue immunostaining with the Olympus® BX 50 multi-head optical microscope was considered positive when the cases were strong and diffuse brown-brown cytoplasmic and nuclear marking (Figure 3) (Figure 4).
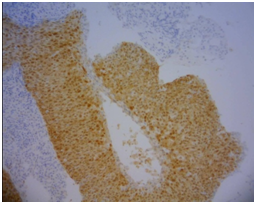
Figure 3 Positive for P16.
Note: Photomicrograph of p16 positive squamous cell carcinoma (brown area). 100x Zoom.
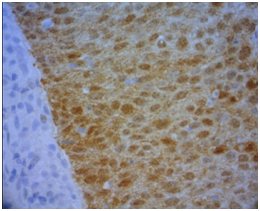
Figure 4 Details from positive p16.
Note: Detail of p16 positivity in squamous cell carcinoma demonstrating strong cytoplasmic and nuclear labeling (brown). 400x Zoom
All results and information obtained were labeled according to data protocol, and then expressed through graphs and tables, developed from Excel spreadsheets. In this study, the chi-square test was used to analyze the difference between the observed result and the expected result. However, this test is limited. When more than ¼ of the expected values in a 2x2 table were less than 5, we used Fisher's exact test and Cramer's V statistic. For tables with variables that had more than two parameters, larger than 2x2, we used logistic regression for dependency analysis.
All the material containing the information was the responsibility of the researchers themselves, ensuring the maintenance of confidentiality and confidentiality.
The present sample consisted of 51 cases, 42 males and 9 females, with ages ranging from 44 to 89 years, average of 61 years and standard deviation of 10.64 years. The most frequent location of the tumors was in the larynx territory, with 22 cases, followed by tongue lesions, 16 cases, 10 in the mouth and 3 in palatine tonsils.
In regard of results in our research, head and neck SCC prevailed in males, which accounted for 42 cases (82.35%). In this group, the most prevalent topography was the larynx, with 19 cases (45%). Tongue tumors accounted for 13 cases (31%), mouth 8 (19%) and tonsils, 2 (5%). On the other hand, in females, there were the same amount of laryngeal and tongue tumors, 3 cases each (33%), two in the mouth (22%) and a single case in tonsils (11%) (Figure 5).
Regarding the histological differentiation pattern, most of the lesions in the sample were keratinized, as shown in Table 1.
Line labels |
Mixed |
Non-keratinized |
Keratinized |
Grand total |
% |
Mouth |
1 |
3 |
6 |
10 |
20% |
Larynx |
6 |
6 |
10 |
22 |
43% |
Tongue |
3 |
5 |
8 |
16 |
31% |
Tonsils |
3 |
3 |
6% |
||
Grand Total |
10 |
17 |
24 |
51 |
100% |
Table 1 Proportion between histological types and injury topography
Among men, the keratinized tumors were 21 (50%), and among the non-keratinized and mixed tumors there was some homogeneity, corresponding to 11 and 10 cases, respectively. Among women, no cases of mixed tumors were found, and the highest prevalence was non-keratinized tumors, making up 66.67% (Table 2).
|
Female |
% |
Male |
% |
Total |
Mixed |
0 |
0 |
10 |
24% |
10 |
Non-keratinized |
6 |
66,67% |
11 |
26% |
17 |
Keratinized |
3 |
33,33% |
21 |
50% |
24 |
Grand Total |
9 |
100% |
42 |
100% |
51 |
Table 2 Distribution of prevalence of histological type of injury and sex
Tumors displaying koilocyte expression predominated in our sample, 29 (56.86%) of 51 cases. In this group of tumors with koilocytosis, the predominance was in laryngeal SCC, with 45%, followed by tongue tumors in which the diagnostic cell was present in 34% of the lesions (Table 3). There is no correlation between these variables at the 5% level. The logistic regression test was used for this calculation; the calculated p-value was 0.329.
|
No |
% |
Yes |
% |
Total |
Mouth |
5 |
23% |
5 |
17% |
10 |
Larynx |
9 |
41% |
13 |
45% |
22 |
Tongue |
6 |
27% |
10 |
34% |
16 |
Tosils |
2 |
9% |
1 |
3% |
3 |
Grand Total |
22 |
100% |
29 |
100% |
51 |
Table 3 Relationship between coilocytosis and topography of injuries
The prevalence of koilocytes occurred in keratinized tumors, with an index of 72%, as shown in Table 4.
Rótulos de linha |
No |
% |
Yes |
% |
Grand total |
Mixed |
4 |
18% |
6 |
21% |
10 |
Non-keratinized |
15 |
68% |
2 |
7% |
17 |
Keratinized |
3 |
14% |
21 |
72% |
24 |
Grand Total |
22 |
100% |
29 |
100% |
51 |
Table 4 Relation between coilocytosis and histological type of injuries
Correlating topography with positivity for p16, the present sample showed no statistically significant relationship (p=0.7821) (Table 5).
|
Mouth |
Larynx |
Tongue |
Tonsils |
Total |
Negative |
6 |
12 |
7 |
1 |
26 |
Positive |
4 |
10 |
9 |
2 |
25 |
Total |
10 |
22 |
16 |
3 |
51 |
Table 5 Relation between p16 biomarking and injury topography
The prevalence of p16 positivity among all lesions was 49.02% (25 cases). Comparing p16 expression and histological type, the highest positivity was in non-keratinized tumors, making up 12 cases (48%). There was statistical significance by Fisher's exact test for this comparison (p=0.03532) (Table 6).
|
Negative |
% |
Positive |
% |
Total |
Mixed |
8 |
31% |
2 |
8% |
10 |
Non-keratinized |
5 |
19% |
12 |
48% |
17 |
Keratinized |
13 |
50% |
11 |
44% |
24 |
Grand Total |
26 |
100% |
25 |
100% |
51 |
Table 6 Relationship between p16 biomarking and histological type of injuries
Relating the presence of koilocytes and positivity for p16 immunoexpression was positive in 25 cases (49%) (Table 7).
Line labels |
No |
Yes |
Grand total |
% |
Negative |
8 |
18 |
26 |
51% |
Positive |
14 |
11 |
25 |
49% |
Grand Total |
22 |
29 |
51 |
100% |
Table 7 Relationship between coilocytosis and biomarking of p16
During the research fifty-one cases of head and neck squamous cell carcinoma were studied in the present series, analyzing the epidemiological profile of the patients and the positivity for p16 protein immunostaining.
Over the recent years, numerous immunohistochemical markers have been studied in the management of oral cavity squamous cell carcinomas, as well as in HPV research in this cancers.10 The p16 protein, which acts as a tumor suppressor protein by competitively inhibiting cyclin-dependent kinesis, is an important example of this new diagnostic strain. It is found in low concentrations in the normal epithelium, and its expression is increased in proliferative and inflammatory processes (Britto, Paula and Sadi, 2014). And the main reason for its study, allowing the analysis of viral infection in these tumor types, is the fact that HPV positive patients have shown a better prognosis,10 as well as a better response to treatment and sensitivity to radiotherapy.17
In the present research, there was a prevalence of head and neck CPB in male patients, with 42 out of 51 patients (82.35%), with a mean age of 61 years, which corroborates the data released by INCA in 2016, in which this index was 71.92%. In addition, this higher prevalence in men is also found in the research conducted by Antonsson17 in Queensland, Australia, which showed a higher number of these tumors in men (74%) with a mean age of 62 years.
Regarding the topography of the lesion, there is a higher concentration of SCC in the books, making a total of 95% for head and neck tumors.1 It is also noticeable in the Australian study by Antonsson,17 which demonstrates tumor involvement of the oral cavity in 36% of tumors. However, in our study, the lesions showed higher prevalence in another location, in the larynx, corresponding to 43% of cases. Possibly, this discrepancy may be related to a different anatomical division performed by the Australian study, which subdivides a larger number of compartments. While our sample was grouped into mouth, tongue, tonsil and larynx, the study sample is classified into mouth, oropharynx, hypopharynx and larynx.
In the present study, it was observed that the cases of keratinized head and neck squamous cell carcinoma are the most prevalent (47.06%), except for women, where a predominance of the pattern of non-keratinized tumors was observed. Regarding this factor, no comparative parameters were found in the literature.
Koilocytosis, which is the morphological expression of HPV infection, which can be noted in cytopathology and histopathology, was evidenced in 29 cases (56.86%) of this sample, predominantly in keratinized tumors. Regarding this factor, no comparative parameters were found in the referential literature.
An Immunoexpression of p16 protein, which is a biological marker related to neoplastic induction by HPV, showed a prevalence of 49.02% in the present series, predominating in non-keratinized tumors and laryngeal localization (19.60%). Regarding this immunostaining, Fischer et al.8 described that among 365 CECCP biopsies, p16 expression was present in 288 cases (78.9%), with 32.05% found in the mouth topography. Quite possibly, this discrepancy from the values found in the literature and our study may be related to the sample size, and to a higher concentration of cases in the mouth and oropharynx topography, which are the most prevalent places in the literature.18–25
Thus, the present study term-paper aims to contribute to a better understanding of the oncogenesis of head and neck squamous cell carcinomas. Notwithstanding its results, it would be interesting to increase the number of cases for greater representativeness of this group of lesions, as well as to enable other studies to compare, for example, the biological behavior and growth of these tumors with their prognosis.26–33
The present term-paper study concludes:
None.
None.
The author and co-authors have no conflicts of interest relevant to this article.

©2019 Sanches, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.