eISSN: 2377-4304


Case Report Volume 5 Issue 3
Department of Obstetrics and Gynaecology, Singapore General Hospital, Singapore
Correspondence: Wai Yen Lee, Department of Obstetrics and Gynaecology Singapore General Hospital Outram Road Singapore 169608
Received: September 20, 2016 | Published: October 24, 2016
Citation: Lee WY, Tan WC, Tan YR, et al. Congenital intracranial space occupying lesion: a case report. Obstet Gynecol Int J. 2016;5(3):340-343. DOI: 10.15406/ogij.2016.05.00160
Congenital intracranial space occupying lesion (SOL) is extremely rare. We present a case of congenital intracranial space occupying lesion diagnosed during ultrasound at 33 weeks gestation. Following delivery by caesarean section at 34 weeks, cranial ultrasound and magnetic resonance imaging (MRI) of the brain suggested primitive neuroectodermal tumour. Parents declined surgery and baby subsequently passed away on day 11 of life. Prognosis of congenital SOL is generally poor. Accurate antenatal diagnosis is crucial to help in counselling and discussion of delivery options or termination of pregnancy.
Keywords: Primitive neuroectodermal tumour, Congenital brain tumour, Intracranial tumour, Congenital space occupying lesion
SOL, Space Occupying Lesion; MRI, Magnetic Resonance Imaging; VDRL, Venereal Disease Research Laboratory; HBsAg, Hepatitis B Surface Antigen; HIV, Human Immunodeficiency Virus; MoM, Multiples of Median; MCA PI, Middle Cerebral Artery Pulsatile Index; IgG, Immunoglobulin G; HC, Head Circumference; AC, Abdominal Circumference
A congenital intracranial space occupying lesion (SOL) is defined as a lesion that is present at birth or first detected in the first few months of life. It is extremely rare, with reported incidence of 0.5 to 1.5% of all cerebral tumours in children.
A 34 year old woman with 2 previous first trimester miscarriages and antenatal history of gestational diabetes well controlled on diet was initially booked at a private hospital for her antenatal follow up. Her first trimester screening was low risk for trisomy and screening scan at 20 weeks showed no fetal anomaly. Antenatal routine screening blood tests, including hepatitis B surface antigen (HBsAg), venereal disease research laboratory test (VDRL) for syphilis and human immunodeficiency virus (HIV) were negative. Her blood group was AB positive with no atypical antibody. She did not have family history of stroke or thrombophilia.
Ultrasound at 32 weeks showed a raised middle cerebral artery peak systolic velocity (MCA PSV) above 1.5 Multiples of Median (MoM). Repeat ultrasound at 33 weeks showed a right brain lesion measuring 5.0 x 3.1 cm. Middle cerebral artery pulsatile index (MCA PI) was below the 5th percentile. Parvovirus immunoglobulin M (IgM) and immunoglobulin G (IgG) were negative. Kleihauer-Betke test did not show any fetal maternal haemorrhage.
Following referral to our unit, ultrasound showed the head circumference (HC) and abdominal circumference (AC) to be on the 5th percentile. A 4cm homogeneous hypoechoic area was seen in the posterior brain (Figure 1). Differential diagnosis included an infarct, haemorrhage or SOL. MCA PI was markedly low at 0.6, but umbilical artery pulsatile index (UA PI) and ductus venosus pulsatile index (DV PI) were within normal limits. Antiplatelet antibody (anti-heparin PF4), lupus anticoagulant and anti-cardiolipin IgM and IgG antibody were not detected. A repeat ultrasound 2 days later showed similar findings.

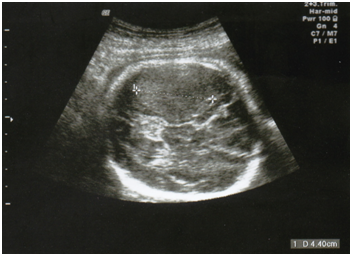
Figure 1 Antenatal ultrasound in transverse plane showing a 4 cm homogenous hypoechoic area in right posterior brain.
In view of possible fetal brain infarct, elective caesarean section was performed at 34 weeks gestation after completion of intramuscular betamethasone. A baby girl was delivered with good Apgar scores of 8 and 9 at 1 and 5 minutes, and birth weight of 2040 g. The newborn received brief positive pressure ventilation (PPV) for 1 min, and remained stable thereafter on room air. She was neurologically intact, moving all 4 limbs equally with normal tone and reflexes, and was able to feed normally. Electroencephalogram (EEG) showed normal continuous activity with no seizures observed. Blood test for coagulation profile showed normal platelet count (170 x109/L), prolonged prothrombin time (PT, 14.3 seconds) and prolonged activated partial thromboplastin time (APTT, 72.3 seconds). Coagulopathy was corrected with fresh frozen plasma.
Ultrasound of the brain on day 1 of life showed a right parieto-occipital hypoechoic mass of 4.8 cm with 2.1 cm hyperechoic density (Figure 2). Magnetic Resonance Imaging (MRI) of the brain showed a large haemorrhagic right temporo-occipital mass measuring 5.6 cm, with early hydrocephalus, suspicious of a supratentorial primitive neuroectodermal tumour (PNET) (Figure 3).

Figure 2 Ultrasound brain done on day 1 of life showed a right parieto-occipital hypoechoic mass 4.8 x 4.0 cm, with 2.1 x 1.2 cm hyperechoic density compressing right ventricle with midline shift to left and abnormal grey-white matter differentiation.
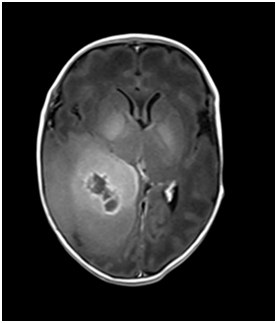
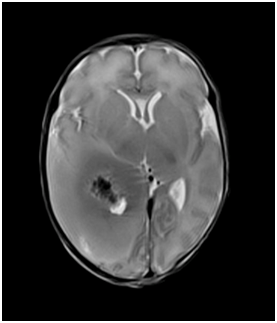
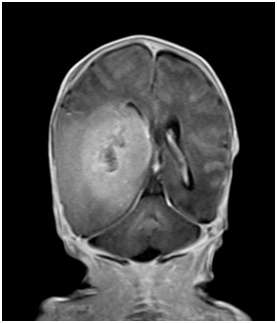
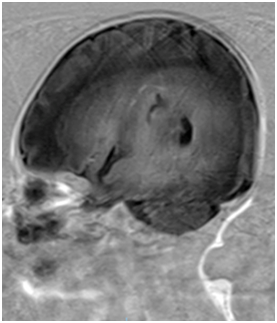
Figure 3 MRI of baby brain showing heterogeneously enhancing large right hemispheric mass involving the ipsilateral temporal and occipital lobes. It measures 5.6 x 3.4 x 4.5 cm. The solid enhancing component shows restricted diffusion, indicative of cellularity. It causes significant mass effect on the adjacent cortical sulci, basal cisterns, body and occipital horn of the right lateral ventricle, resulting abnormal dilatation of both the temporal horns and occipital horn of the left lateral ventricle, indicative of early hydrocephalus. Midline shift was noted to the left side (2 mm). There was an intratumoural haemorrhagic component measures 2.0 x 4.0 cm. No significant surrounding edema was seen.
She was subsequently transferred and counselled for surgery by the paediatric neurosurgeon. Parents declined surgery in view of poor surgical outcome. The option of biopsy for definitive diagnosis and chemotherapy was discussed but declined. The parents opted for comfort care and were taught nasogastric tube insertion and basic cardiopulmonary resuscitation (CPR) before the baby was discharged against advice. On day 11 of life, the baby was admitted with vomiting and lethargy. Computerized tomography (CT) brain (Figure 4) showed marked increase in the midline shift and baby passed away the same night, with intracranial tumour contributed by tumour haemorrhage confirmed as the cause of death. No post-mortem was performed.
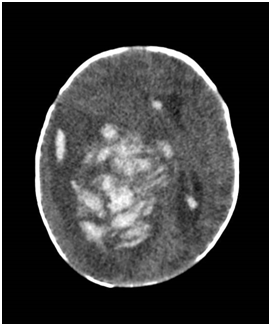
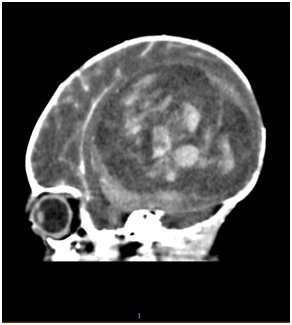
Figure 4 CT of baby brain showing a heterogenous enhancing mass that is occupying right cerebral hemisphere, measuring 8.0 x 5.3 x 7.1 cm, causing significant midline shift towards the left. There were areas of hyperdensity showing intraventricular extension of haemorrhage to the right frontal horn, posterior horn and third ventricle.
A congenital intracranial space occupying lesion (SOL) is defined as a lesion that is detected during pregnancy or detected in the first three months of life.1 Some affected infants may develop symptoms in the first year of life. It is extremely rare, with reported incidence of 0.5 to 1.5% of all childhood brain tumours.2 Among those tumours presenting in the first year of life, only 18% are diagnosed before delivery.3 Differential diagnosis of congenital intracranial SOL include tumours that are either solid (Table 1) or cystic (Table 2), haemorrhage or infarct and brain malformations.
|
Solid SOLs |
Incidence |
|
Teratoma |
50% |
|
Glioma (Astrocytoma, Ependymoma, Oligodendroglioma) |
25% |
|
Primitive Neuroectodermal Tumour (PNET) |
25% |
|
Craniopharyngioma |
|
|
Choroid plexus Papilloma |
|
|
Neuroblastoma |
|
|
Gangliocytoma |
|
|
Meningeal sarcoma |
|
|
Capillary Haemangioblastoma |
|
|
Hypothalamic Hamartoma |
Table 1 Types of congenital solid space occupying lesion and incidence2
The commonest solid tumours seen are teratomas which accounts for half of all solid congenital intracranial tumour. Teratomas are benign, and arise from migrating foci of totipotent cells that have escaped the organizing influences associated with early development. Teratomas are generally mixed solid and cystic.4 If cystic component predominates, it may be difficult to distinguish from cystic SOL such as arachnoid cyst. Calcification may be seen in teratomas but it is not characteristic as may be present in other histological types such as primitive neuroectodermal tumour. Teratomas are usually located in the midline, usually from the pineal region, or can be located in suprasella region, ventricles and posterior fossa.
Gliomas, the second most common tumour, are tumours of the neuroepithelial tissue, arising from glial cells. For example, astrocytoma, ependymoma and oligodendroglioma. Unlike teratomas which can be mixed solid cystic, gliomas are usually large solid tumours involving one or more cerebral hemispheres and it may displace the lateral ventricles.5 Astrocytoma is the commonest glioma and can range from benign to malignant.
Other differential diagnosis of congenital solid tumour includes rare cases of primitive neuroectodermal tumour (PNET), choroid plexus papilloma, neuroblastoma, and gangliocytoma. PNETs are high grade malignant small-cell tumour and are thought to derive from the neural crest. It is characterized by early recurrence, metastasis, and high mortality.3 These tumours are frequently supratentorial, involving the cerebral hemispheres and pineal regions. On MRI scan, they are usually large heterogeneous masses that are hypointense on T2 and give restricted diffusion consistent with high cellularity. Perifocal edema may be scanty in spite of the huge size. In our case, MRI of the baby brain showed heterogeneous large right hemispheric mass with solid enhancing component showing restricted diffusion, indicative of cellularity. It causes significant mass effect, resulting in early hydrocephalus and midline shift. No significant surrounding edema was seen. Diagnosis is challenging with imaging alone and histology is usually needed for definite diagnosis. Because it is often deep-seated, PNET often poses significant challenges to safe resection. It is highly aggressive and very often therapy resistant in young children.6 Histology diagnosis was not possible in our patient as parents declined surgery or biopsy, and no post-mortem was performed. Molecular genetic studies of PNET are limited by the rare incidences and recurrence in subsequent pregnancies is unknown.
Choroid plexus tumour is usually located in the lateral ventricle, and less frequently in the third or fourth ventricles. It can be a benign papilloma or carcinoma. Unfortunately, colour Doppler imaging does not allow differentiation between benign or malignant. It is associated with hydrocephalus caused by overproduction of cerebrospinal fluid or by obstruction of the foramen of Monro.7
Prognosis of congenital intracranial solid tumour is generally very poor. Mortality rate for teratoma, ependymoma and PNET is about 90%, due to the rapid growth. The outcome is usually either stillbirth or death shortly after birth. Choroid plexus papilloma has the best prognosis among congenital intracranial solid tumour (Table 2).1
|
Cerebral tumours |
Mortality Rate |
|
Ependymoma |
91% |
|
Teratoma |
88% |
|
PNET |
88% |
|
Craniopharyngioma |
76% |
|
Astrocytoma |
64-68% |
|
Choroid plexus Papilloma |
27% |
Table 2 Main congenital solid intracranial tumours and mortality rate1
Differential diagnoses of cystic congenital SOLs include choroid plexus cysts (CPC), arachnoid cysts, and porencephalic cysts (Table 3). Choroid plexus cysts are sonolucent cysts within the choroid plexus of the lateral ventricle. Incidence of CPC is estimated to be 1% and it may be associated with trisomy 18 and 21. CPC may be unilateral or bilateral and regress spontaneously by 28 weeks.8,9 Arachnoid cysts, which develop from arachnoid membrane, are benign accumulation of clear fluid between the dura and brain substance and usually do not communicate with the ventricular system. They have a male predominance and are mostly supratentorial. Porencephalic cysts are usually unilateral and communicate with the ventricular system.9
|
Cystic SOLs |
|
Choroid plexus Cyst |
|
Arachnoid Cyst |
|
Porencephalic Cyst |
|
Open-lip types of Schizencephaly |
|
Interhemispheric Cyst |
|
Posterior Fossa Cyst |
Table 3 Types of congenital cystic space occupying lesion2
Other differential diagnoses of congenital SOLs include antenatal spontaneous intracranial haemorrhage occurring in the presence of coagulation factor deficiency, congenital intracranial vascular malformations such as capillary haemangioma and arteriovenous malformations such as aneurysmal malformation of the vein of Galen.10
Clinically, congenital SOL may result in spontaneous intracranial haemorrhage in utero, hydrocephalus, macrocephaly, intrauterine growth retardation and stillbirth. Large SOLs may be lead to fetal cardiac failure, hydrops fetalis and polyhydramios due to swallowing disorder. Congenital SOLs is reported to be associated with fetal cardiac and urinary malformations in about 14% of cases.2
Antenatal detection of congenital SOLs is usually incidental during routine antenatal ultrasound at 20 weeks gestation to exclude structural anomalies, during routine growth scan or ultrasound performed for decreased fetal movement or polyhydramios. The median gestational age at the time of diagnosis in these cases was 31.5 weeks.11 However, this varies depending on the underlying pathology. Differential diagnosis of congenital intracranial SOL is often difficult and fetal MRI is a valuable complimentary tool. Final diagnosis is usually confirmed with histological examination.
The management and counselling of fetal intracranial tumours is dependent on diagnosis and gestational age. An accurate antenatal diagnosis is important to facilitate counselling, mode and timing of delivery. Congenital SOL with poor fetal prognosis diagnosed in early pregnancy may be offered option of termination of pregnancy. If delivery is planned, caesarean section may be needed due to risk of cephalopelvic disproportion. Baby born with intracranial tumour may be offered surgical excision or maximum debulking followed by chemotherapy or radiotherapy. There is no definitive trial data on which treatment has the better outcome but generally radiotherapy is avoided if possible in a developing brain.11
Prognosis of congenital SOLs is generally poor and the diagnosis has a tremendous impact on the family. However, early and accurate diagnosis, timely delivery and management in a tertiary centre with Maternal Fetal Medicine unit, neonatal support facilities and paediatric surgical input are essential to ensure optimal perinatal and neonatal care of a rare condition with guarded prognosis.
None.
None.

©2016 Lee, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.