eISSN: 2377-4304


Research Article Volume 2 Issue 2
1Vincent Department of Obstetrics and Gynecology, Harvard Medical School, USA
2Department of Environmental Health Sciences, University of Michigan School of Public Health, USA
Correspondence: John C Petrozza, Division of Reproductive Medicine and IVF, Vincent Department of Obstetrics and Gynecology, Massachusetts General Hospital, 55 Fruit Street, Boston, Massachusetts, 02114, USA, Tel (617) 726-6942, Fax (617) 724-7530
Received: December 04, 2014 | Published: March 9, 2015
Citation: Petrozza JC, Souter I, Rein MS, et al. Clinical value in IVF: cycle characteristics associated with gonadotropin dose change in IVF patients using low responder protocols. Obstet Gynecol Int J. 2015;2(2):43-47. DOI: 10.15406/ogij.2015.02.00028
Objective: To identify patient and cycle characteristics that influence the frequency of monitoring events and gonadotropin dose change during follicular monitoring of an IVF cycle using a low responder protocol.
Design: Retrospective cohort study
Settings: Academic medical center
Patients: Infertile women undergoing IVF using a low responder protocol (GnRH agonist Flare or GnRH antagonist)
Intervention: Retrospective chart review
Main Outcome Measures: Gonadotropin dose change, initiation of GnRH antagonist, and administration of hCG
Results: Gonadotropin dose change in low responders rarely occurs in the first five days of gonadotropin stimulation in an IVF cycle. There were no baseline patient characteristics associated with change in medication management. After three days of gonadotropin stimulation, serum estradiol level >300 pg/ml predicted any change in medication management for the next three days with 100% and 92% sensitivity for the Flare and Antagonist protocols, respectively.
Conclusion: For patients receiving a low responder protocol, there are early cycle characteristics associated with change in gonadotropin dose during the cycle. Monitoring intensity can likely be modified according to these criteria to reduce the total number of interventions.
Keywords: clinical value, follicular monitoring, gonadotropin dosing, low responder protocols
E2, estradiol; CD, cycle day; IVF, in vitro fertilisation; HCG, human chorionic gonadotropin.
Careful monitoring of estradiol (E2) levels and ultrasound characteristics during an IVF cycle is paramount to ensuring an adequate response to gonadotropin stimulation, while mitigating the risk of hyper-response. Through these monitoring interventions, gonadotropin dosing can be appropriately titrated, start of a GnRH antagonist properly timed, and hCG accurately administered. Clinical experience guides the frequent decisions around monitoring frequency and gonadotropin dose changes. Certain protocols, such as the GnRH-antagonist, will require more frequent monitoring in order to identify specific mid-cycle events. Appreciation of particular trends has led to creation of stimulation protocols that require less intensive monitoring.1,2 There are likely particular patient characteristics that would allow accurate prediction of monitoring intervals. Rather than monitoring on “regular” intervals, an objectively-designed monitoring pattern could reduce the number of interventions and deliver comparable clinical outcomes. A reduction in the number of interventions would impact a number of fixed and variable costs involved in the monitoring process. This study aims to explore patient and cycle characteristics that influence the frequency of monitoring events and gonadotropin dose change in patients using a low responder protocol. By identifying parameters associated with monitoring frequency and gonadotropin dose changes, we intend to develop a framework for best-practice approach.
The study was approved by the Partners Healthcare Institutional Review Board. Data from all IVF cycles leading to oocyte retrieval was obtained (01/01/2009-12/312009). Only patients receiving a low responder protocol (either the follicular GnRH-agonist flare or GnRH-antagonist protocol) were included in this study. All patients had a baseline ultrasound performed on designated cycle day (CD) two or three. A follicular ultrasound and serum E2 were obtained at all subsequent monitoring visits, and gonadotropin dosing was adjusted, as needed. The second monitoring event was performed on CD6. hCG was administered with at least three dominant follicles (>15mm) present and E2 ≥600pg/ml.
Information was collected on baseline patient characteristics (age, day-3 FSH, BMI, antral follicle count, history of prior cycle) and the number of days in cycle. Data was collected on the CD of the ultrasound, number of follicles seen (total, and ≥14mm), E2 level, day of GnRH-antagonist initiation and hCG- administration, and on whether gonadotropin dose increased, decreased, or remained unchanged. A management change was defined as any change in gonadotropin dosing, initiation of the GnRH-antagonist, or administration of hCG. Subgroup analysis of the actual data was used to estimate variable measurements for modeling different GnRH-antagonist start criteria (fixed E2 or cycle day). Cost estimates were based on 2012 Medicare reimbursement rates for a follicular ultrasound ($92.02) and serum E2 ($39.32).3
Primary outcomes included: changes in gonadotropin dosing, GnRH-antagonist initiation, and hCG administration. Chi-square, t-test and Wilcoxon rank-sum test were used as appropriate (p≤0.05 was considered significant).
Complete data was available for 3,871 ultrasounds from 689 IVF cycles in 393 patients during this time period (22% flare, and 14% antagonist cycles).
GnRH-agonist flare cycles
Ninety-nine patients using the flare protocol had 760 ultrasounds in 146 cycles (5.2±0.9 ultrasounds/cycle [range: 3-8], average cycle duration: 11.3±1.8 days [8-16]). Figure 1 shows the distribution of ultrasounds performed in flare cycles and how monitoring relates to management change. From CD6 to CD8, one management change occurred for every 9.9 monitoring events. There were 24 dose decreases during this interval, and on CD8, hCG was administered to 5 patients. Only one live birth occurred in these 20 unique cycles. There was no difference in baseline patient characteristics for those who did and did not have a management change during this period.
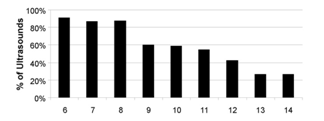
Figure 1B Percent of ultrasounds by cycle day that do not result in a management change (gonadotropin dose change or administration of hCG).
Figure 1 GnRH Agonist Flare Cycles.
A CD6 estradiol level of 300pg/ml was 100% sensitive and 72.1% specific for identifying those who had a gonadotropin dose adjustment or hCG-administration during this time period. Of 138 CD6 monitoring events, 51 cycles (37.0%) had an E2≥300pg/ml. Of those, 17 cycles (33.3%) had a management change prior to CD9. No differences in baseline characteristics were identified between the groups that had a CD6 E2 ≥300pg/ml to those with lower E2 levels. Cycles with a CD6 E2<300 pg/ml were significantly less likely to have a dose change anytime during their cycle compared to those with higher E2 levels (p<0.0001). Additionally, the higher estradiol population showed a greater average peak E2, and follicle number (total and ≥14mm) on the day of hCG-administration, and a lower average number of ultrasounds and day of hCG-administration (Table 1).
| Estradiol > 300 ng/ml | Estradiol <300 ng/ml | |
|---|---|---|
Peak Estradiol (ng/ml) |
2311 ± 1130 (797-5702) |
1565 ± 713 (374-3811) |
Total Number Follicles* |
11.5 ± 4.1 (4-23) |
9.6 ± 3.7 (2-25) |
Number ultrasounds** |
4.8 ± 0.8 (3-7) |
5.5 ± 0.9 (4-8) |
HCG administration (CD) |
9.7 ± 1.0 (8-12) |
12.2 ± 1.5 (9-16) |
Table 1 Characteristics of GnRH Agonist Flare cycles according to cycle day six serum estradiol levels (mean ± SD (range))
*Denotes statistical significance at p=0.02 level.
**Denotes statistical significance at p< 0.001 level.
In patients with CD6 E2 ≥300pg/ml, from 239 total monitoring events, there were 33 gonadotropin dose changes (29 decreases) in 22 unique cycles and individuals, giving an average of one gonadotropin dose change per 7.2 monitoring events. There was no difference in baseline patient characteristics or CD6 E2 level between those who did and did not have a management change. There was a significantly higher total number of follicles seen in cycles with a management change (11.5±4.9 versus 9.0±3.2, p=0.05).
In patients with CD6 E2 levels <300pg/ml, there were 473 monitoring events, with 20 gonadotropin dose changes (19 decreases, all after CD8) in 13 unique cycles and individuals. hCG was not administered in any of these cycles before CD9, (one gonadotropin dose change per 23.7 monitoring events). Gonadotropin dose change occurred on average on CD: 10.9±1.4 (9-13). Those requiring a dose decrease had peak E2 levels: 2588±893pg/ml (1292-3811). Compared to all cycles with a day-6 E2<300pg/ml, those requiring a dose decrease had a significantly higher number of follicles (total and ≥14mm: 12.5±0.7 vs. 9.8±0.4, p=0.01, and 6.5±0.7 vs. 4.7±0.3, p=0.02, respectively), although there was no significant difference in peak E2 levels.
GnRH antagonist cycles
Following a conventional monitoring approach, 555 ultrasounds were performed for 93 antagonist cycles in 76 unique individuals (6.0±1.0 ultrasounds/cycle [range: 4-9], average cycle duration: 11.6±1.6 days [8-17]). The GnRH-antagonist was started on CD 9.0±1.6 (6-15) with a mean E2: 874±400 pg/ml (230-2019), (Table 2).
| Actual | Fixed Estradiol | Fixed Cycle Day 8 | Fixed Cycle Day 9 | |
|---|---|---|---|---|
Antagonist Start Day |
9.0 ± 1.6 |
10.1 ± 1.9** |
-- |
-- |
Estradiol level (ng/ml) |
874 ± 400 |
1144 ± 236** |
715 ± 482** |
1051 ± 592 |
Follicles ≥14mm |
0.9 ± 1.4 |
2.8 ± 2.6** |
0.6 ± 1.2* |
2.3 ± 2.7** |
Table 2 Characteristics of GnRH Antagonist cycles based on actual cycle data or subgroup analysis of actual data (mean ± SD (range))
*Denotes statistical significance at p=0.04 level, relative to actual levels.
Denotes statistical significance at p< 0.001 level, relative to actual levels.
Clearly, a large number of ultrasounds were performed between CD6 and CD10 that did not result in gonadotropins dose changes in Figures 2(A &B). Most of these ultrasounds were being performed to optimally time the GnRH-antagonist initiation, as indicated by the large number of antagonist starts from CD8 to CD10 (Figure 2C). From CD6 to CD8, one management change occurred for every 3.7 monitoring event; excluding antagonist starts, one management change occurred every 12.1 monitoring events.

Using a fixed cycle day GnRH-antagonist start provided a closer and more conservative fit than using a fixed E2 level in terms of mimicking conventional monitoring. Statistical comparisons between antagonist start day, serum E2 levels, and the number of follicles ≥14mm were made between the cycles based on the actual and predicted antagonist start day (Table 2). The fixed E2 level start at 1000 pg/ml produced a significantly later antagonist start in the cycle at a higher E2 level with more follicles ≥14mm.
For the fixed GnRH antagonist cycle day start, comparisons were made between a fixed CD8 or CD9 start, and the actual monitoring data (Table 2). Data from a CD9 start day showed a similar E2 level at initiation of the GnRH-antagonist, but significantly more follicles ≥14mm. A CD8 start was more optimal from a conservative perspective: both E2 levels and number of lead follicles were less than that observed in the actual data. Several details require additional consideration if using a fixed CD8 GnRH-antagonist start. First is the number of cycles that had a GnRH-antagonist start before CD8 (15/93 or 16%). E2 level on the GnRH- ntagonist start day for these fifteen cycles was 640±280 pg/ml (range: 242-1271) with 0.2±0.4 follicles ≥14mm (one cycle resulted in a live birth, three in biochemical conceptions and eleven in no pregnancy).
Secondly, we analyzed how often gonadotropin dose changes being made using intensive monitoring would be missed if monitoring was not resumed until after the GnRH-antagonist start. Eight unique individuals (8.6%) had at least one gonadotropin dose change prior to CD9, seven of which were a gonadotropin decrease on CD6 (peak E2: 3003±882 pg/ml). Lastly, we analyzed how timing of hCG-administration would be impacted if monitoring did not resume until CD9. Two cycles had hCG administered on CD8 without prior dose changing (peak E2: 990 and 2359 pg/ml, with 3 and 5 follicles ≥14mm, resulting in a biochemical pregnancy and a negative hCG, respectively).
A CD6 serum E2 could potentially identify patients undergoing rapid, early recruitment or greater than expected response to gonadotropins. Using an E2 ≥300pg/ml would have identified 30 patients requiring further evaluation. All eight patients who had a gonadotropin dose change and both patients undergoing hCG- administration prior to CD9 were in this population. Thirteen of 15 patients who initiated the antagonist prior to CD8 were included by these criteria. However, one of the missed cycles resulted in live birth. This serum E2 ≥300pg/ml would provide a sensitivity and specificity for identifying a change in management of 92.0% and 89.7%. There were no differences in baseline patient characteristics for those who did and did not have a management change when E2 was ≥300pg/ml.
There is a cost that influences the total expense of an IVF cycle. Direct costs include staffing, space, supplies, laboratory expense, and amortization of ultrasound and equipment. Indirect costs include patient time (away from their work) and travel. In a future foreshadowed by detailed scrutiny of clinical value in healthcare, these are certainly important considerations. Anticipating them will allow tailoring of monitoring regimens, such that they continue to deliver robust clinical outcomes at optimized cost.
Clinical value is a metric of increasing interest in this era of cost reform.4–11 This study has focused on this issue of reducing the cost of care delivery, while maintaining comparable clinical outcomes. Just as careful attention is directed towards protocol selection, the monitoring regimen can be similarly considered. For the optimal approach, monitoring intensity/cost can be modified according to the number of serum hormone measurements and number ultrasounds.
Knowing of patient characteristics that influence the frequency of monitoring and likelihood of dose change will allow the stratification of patients into monitoring regimens of varying intensities. We were not able to identify a priori patient characteristics associated with changes in management in flare or antagonist cycles. As an alternative to baseline characteristics, early events in a stimulated cycle can be also used to stratify monitoring intensity. Low responders have the appeal of reduced variability in gonadotropin dosing compared to the general IVF population.
Flare cycles had a large number of ultrasounds performed from CD6 to CD8, with rare changes in management. As only one live birth resulted from 27 management changes in 20 unique cycles (5%), it is debatable whether it is necessary to isolate these cycles. Nevertheless, using a estradiol threshold of 300 pg/ml on CD6 is a practical alternative, as there is a large difference in ratios of monitoring events that are performed per management change (7.2 events for E2 >300pg/ml vs. 23.7 events for lower levels). Additionally, a CD6 E2 ≥ 300pg/ml provided 100% sensitivity in identifying cycles in which management changes occurred prior to CD9.
A potentially more cost effective consideration would be to administer hCG solely according to follicular measurement parameters, without the use of serum E2. Within the overall population of CD6 E2 <300pg/ml, those that did require a dose change were compared to those that did not. A gonadotropin step-down would be an appropriate consideration in the terminal portion of stimulation with numerous follicles (≥8 total follicles, based on the minimum range in the group with later management changes).
The above data suggest a method whereby the flare population is stratified according to CD6 E2 level. Using the assumptions that those with E2 >300pg/ml maintain the same monitoring intensity, while those with lower levels would forgo additional monitoring until CD9, at which point they would resume the original monitoring intensity, this approach could eliminate 136 ultrasounds (29.4%) and 56 E2-measurements, amounting to a reduction in cost of $14,716.64. Figure 3 illustrates our proposed flare monitoring protocol.
For antagonist protocols, we currently use two criteria to start the GnRH-antagonist: serum E2 level or a lead follicle size. Monitoring events were occurring in these cycles to adjust gonadotropin dosing, accurately time the start of the GnRH-antagonist, and administer hCG. Between CD6 and CD8, one of these three events occurred for every 7.7 monitoring episodes. Using a fixed level of 1000pg/ml provided a poor fit, as it initiated the antagonist significantly later than with the conventional approach, which could predispose the cycle to either escape ovulation or early luteinization. A lower E2 threshold (900pg/ml), may be an alternative to consider.
26.8% of the cycles had a management change prior to CD9, and it may be important to capture this segment of the population, although they often reflect an effort to salvage cycles with rapid recruitment of a small number of follicles. To address these concerns, it is reasonable to obtain a CD6 E2 level, using 300pg/ml as a threshold indicator for additional monitoring. Using this cutoff, 32% of cycles would require additional monitoring, and there would be accurate identification of management changes. Figure 4 illustrates our proposed antagonist monitoring algorithm. A fixed CD8 GnRH-antagonist start is appealing in that it requires less intensive monitoring, closely approximates the actual data, and represents a more conservative approach (Table 2).
Eliminating all monitoring from the baseline until after CD8 would result in the exclusion of 194 (35.0%) ultrasounds, and the same number of E2 measurements. Conservatively, if ultrasound monitoring and E2 measurements proceeded according to conventional monitoring pattern, this would result in a reduction in cost of $25,479.96.
Although reduced costs by these algorithms are static assessments, spin-off benefits would likely include improved patient satisfaction and distribution of resources. Optimistically, downstream monitoring density may also be reduced. Not included in this modeling is the cost of failed cycles using the new system, if it proves suboptimal. At a cost of approximately $10,000 per IVF cycle, a small number of failed cycles would quickly off-set the cost-savings of a more efficient stimulation protocol.12Prospective monitoring would elucidate how these methods perform in their ability to reduce costs and maintain clinical outcomes.
Caution is appropriate in application of these methods. Our population was specifically low responders. Further, these data reflect a uniform approach used within a single center, and there is considerable variability between centers in gonadotropin dosing, and the tolerance of E2 levels and number of recruited follicles.
The aim of this study is to identify methods of limiting over-utilization, which increases costs without improving outcomes. Often, IVF monitoring develops from a combination of experience and habit, although this can potentially be streamlined according to a process and outcomes-driven approach. Multiple other areas within medicine, previously considered too inherently variable, have already been successfully converted into best-approach models. Though achieving exceptional success rates is the foremost concern, optimizing work-flow and resource-allocation will be a necessity for the economic viability of the healthcare system and all practices within it, regardless of size. Many indicators predict that the practices most effective at accomplishing this will harness a competitive advantage going forward.7–10
None.
The authors declare there is no conflict of interests.

©2015 Petrozza, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.