eISSN: 2377-4304


Research Article Volume 14 Issue 1
Universidad de Buenos Aires, Argentina
Correspondence: Abate Camila Rosa, Universidad de Buenos Aires, Argentina, Tel +54 9 11 32433250
Received: February 18, 2023 | Published: February 27, 2023
Citation: Abate CR, Urey GK, Costa MF, et al. Clinical-pathological characteristics and management of fusocellular tumors. Our experience. Obstet Gynecol Int J. 2023;14(1):31-35. DOI: 10.15406/ogij.2023.14.00689
Introduction: Fucocellular tumors encompass a spectrum of very rare entities. Prognostic factors for the risk of relapse and overall survival such as tumor size, surgical margin, histological strain, and stromal growth are described.
Materials and methods: Observational, analytical and cross-sectional study, which studied patients with a diagnosis of fusocellular tumor, treated in the Breast Pathology Service of the Marie Curie Municipal Oncology Hospital (HOMC) of Buenos Aires during the period 2003 to 2022. Quantitative variables were used and analyzed with measures of central tendency. For the comparison of these variables, the Chi 2/Fisher exact test was used.
Results: A total of 58 patients with diagnosis of spindle-cell lesions were recruited, including Filode tumors, sarcomas, hamartomas, adenomyoepithelioma, aggressive fibromatosis, gigantocellular granuloma, fusocellular lesion.
Conclusion: It was observed that early age (<40 years) is not a risk factor for relapse, with an increase in this index in patients with tumor size > 5 cm. The percentage of relapses was not modified when conservative surgeries vs mastectomies were compared.
Keywords: fusocellular tumors, phyllodes tumors, breast sarcomas
Spindle cell tumors are characterized as very rare lesions that span a wide spectrum of entities. They occur in skin, soft tissue and breast tissue, exhibiting the latter component myoepithelial and/or stromal. Its diagnosis is fundamental, since the management and prognosis vary between the different entities of this tumor group.1
The study of these tumors is complex because it constitutes a heterogeneous group of anatomical-clinical lesions, with different variants and morphological subtypes.
Different classifications are described for this type of tumors, dividing into low-grade, borderline or high-grade lesions in relation to their morphology. According to the presence of epithelial component are classified into biphasic lesions (fusiform and epithelial component: Hamartoma, adenomyoepithelioma, PASH, fibroadenoma, benign phyllodes, borderline, malignant; metaplastic carcinoma) or monophasic (spindle-cell component only: desmoid fibromatosis, nodular fasciitis, myofibroblastoma, neural tumors, lipomatous tumor, metaplastic carcinoma similar to fibromatosis, sarcomas, metastasis) (Figure 1). Whereas, for histological diagnosis, the World Health Organization classification of 2020 soft tissue tumors is used.1,2
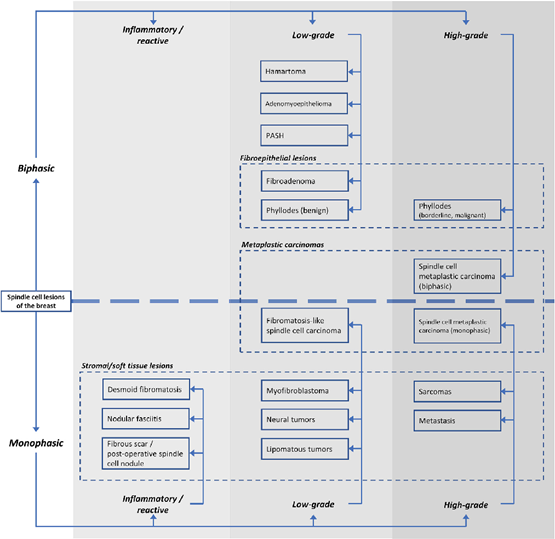
Figure 1 Histological classification of fusocellular lesions.1
Prognostic factors have been associated with age of presentation, rapidly growing lesions, size, type of surgery, state of surgical margins, heterologous component, stromal growth and addition of radiant adjuvancy.
The diagnosis and treatment of this pathology is based on case reports and retrospective series.
There is no consensus regarding treatment, with complete excision being the treatment of choice. In selected cases, the possibility of the study of axillary nodes is considered. The indication of radiotherapy and/or chemotherapy is controversial and they are reserved for special cases.
Given the low frequency of this type of injury, it is important to identify and report cases of these patients, in order to obtain more information that allows the correct treatment of them.
General objective
Describe clinical and pathological characteristics of patients diagnosed with spindle-cell lesions.
Specific objectives:
Design: Observational, analytical and cross-sectional study, with retrospective data collection.
Population and sample: Patients diagnosed with fusocellular tumors were studied and treated at the Breast Pathology Service of the Marie Curie Municipal Oncology Hospital (HOMC) in Buenos Aires during the period 2003 to 2022.
Eligibility Criteria: Patients diagnosed with biphasic tumor, fusocellular tumor with double reading, excluding fibroadenomas. Those tumors that met the histological criteria of the WHO classification and the immunohistochemical labeling characteristic of this group were considered as spindle-cellular lesions.
Method: Patients who met the inclusion criteria were found from the database of the Pathological Anatomy Service. Then information was obtained from physical medical records, which was documented in a flat database for further analysis.
Demographic data (age, personal, gyno-obstetric and family history) and clinical data (clinical stage of onset, anatomopathological characteristics of tumors, cancer treatment and follow-up) were collected.
The staging of patients (borderline-malignant phyllodes, sarcomas) was performed according to the TNM classification of the American Joint Committee on Cancer (AJCC 8 edition 2017). Anatomopathological characteristics of the tumors were analyzed as histological grade and type.
Disease progression or relapse were considered to those patients who at follow-up had lesions compatible with locoregional recurrence (RL) or at a distance, evidenced both in physical examination, complementary studies and / or pathological anatomy.
It was defined as disease-free survival (SLE) at time in months from the date of histological diagnosis to relapse or death, and overall survival (SG) from the date of diagnosis to death due to disease. Relapse or death of patients were determined as follow-up events.
Statistical analysis
The quantitative variables were analyzed with measures of central tendency with their respective dispersions according to the distribution of the variable to be studied, being compared through parametric/non-parametric tests as appropriate. Categorical variables are presented as absolute and relative frequencies (percentages). For the comparison of these variables the Chi 2/Fisher exact test was used according to the conformation of the double input table. STATA 14.0 was used as statistical analysis software.
Ethical aspects of research
The present work is contemplated under the supervision of the Ethics Committee of the Maria Curie Oncology Hospital. The principle of the right of the patient to protection against the invasion of their privacy is ratified and defended by processing all personal information with absolute confidentiality. The study was carried out in accordance with the National Law of Protection of Personal Data (Habeas Data Law). The personal data of patients are used exclusively to achieve the objectives of the study. In the data record, the confidentiality of personal data is guaranteed by identifying each patient with a number that is not linked to data such as ID, name and medical history.
96 patients diagnosed with fusocellular tumor in the period between 2003 and 2022 were recruited in the Breast Pathology Service of the Maria Curie Oncology Hospital. 42 patients were excluded due to lack of availability of medical records.
The study included 54 patients with a diagnosis of fusocellular tumor. The mean age at diagnosis was 43.4 years (Standard Deviation: 12.6) and the median of 44 years (range 16-70). In sex distribution, 52 patients were female and 2 male.
The characteristics of the patients included in this study are detailed in Table 1 below.
Inherited-family background |
|
None |
47% |
Ca. of breast |
16% |
Leukemia |
5% |
Ca. of cervix |
5% |
Ca Ovarian |
4% |
Ca larynx |
4% |
Ca stomach |
4% |
Other background |
15% |
Personal background |
|
None |
86% |
Serous cystadenoma |
5% |
Ca. of breast |
2% |
Ca. of cervix |
2% |
Other background |
5% |
Menarche |
12 (2-18) |
Gestas |
2 (0-8) |
Breastfeeding |
|
They did not breastfeed |
18% |
Less than 1 year |
20% |
More than 1 year |
62% |
MAC |
|
They did not use |
41% |
Barrier |
40% |
ACO |
4% |
DIU |
9% |
Injectable |
6% |
Smoking |
10% |
DBT |
0% |
Obesity |
6% |
Table 1 General characteristics of the population (N=54)
Phyllodes tumor was the most frequent histological strain in the study group (n 39), followed by sarcomas (n 8) (Table 2).
Spindle-cell lesions |
N° 54 |
T.Filodes |
|
High Grade |
12 |
Borderline |
26 |
Low Grade |
1 |
Sarcomas |
8 |
Adenomyoepithelioma |
2 |
Hamartoma |
2 |
Aggressive fibromatosis |
1 |
Fusocellular neoplasia |
1 |
Gigantocellular granuloma |
1 |
Table 2 Incidence of fusocellular tumors
The usual clinical presentation was the unilateral palpable nodule in 46 patients (85.18%), of whom 3 presented rapid and painless growth. The remaining cases were presented as screening study (11.11%), palpable axillary formation (1.85%), bilateral gynecomastia (1.85%).
The tumor size in the surgical specimen presented a mean of 6.41 cm (range 1-15cm). When performing a subgroup analysis, it is observed that according to the TNM classification, the presentation by stage of sarcomas was 3 patients with stage IIIA, 1 stage IIIB, 2 stage IA, 2 stage II.
The entire population was studied with mammography and breast ultrasound. The usual mammographic presentation for these lesions was as a high density nodule, while ultrasonically presented as heterogeneous nodules, lobulated, with regular margins. Only 4 patients had breast nuclear magnetic resonance, presenting this pathology more frequently by this method, as heterogeneous mass or non-mass enhancement.
The diagnosis was made by histological analysis of the lesions. Trucut biopsy was performed in 37 patients (68.51%), vacuum biopsy in 12 patients (22.22%), incisional biopsy in 4 patients (7.41%) and excisional biopsy in 1 pacient (1.85%).
The histological types found in the surgical piece were: 26 low-grade TF (48.15%), 12 high-grade TF (22.22%), 1 borderline TF (1.85%), 8 sarcomas (2 fusocellular, 1 cystosarcoma, 1 leiomyosarcoma, 1 fibrosarcoma, 1 liposarcoma, 1 carcinosarcoma, 1 semi-differentiated angiosarcoma), 2 hamartomas, 1 aggressive fibromatosis, 2 adenomyoepithelioma, 1 fusocellular lesion, 1 gigantocellular granuloma (Table 3).
Histological types |
Subtypes |
Cases |
Immunohistochemistry |
Fusocellular neoplasia |
1 |
Actin: Positive. Ki67: 15%. CKAE1AE3:NEG; RE:NEG; RP:NEG; p53:NEG; HER2:NEG; CK8:NEG; CK18:NEG; CK5;NEG; CK6:NEG; Demine:NEG; p63:NEG; High weight Cytokeratin: NEG |
|
Aggressive fibromatosis |
1 |
Desmin:positive; AML:positive; Bcatenin: Positive. Keratin:NEG; p63:NEG; RE:NEG; CD34: NEG; S100:NEG |
|
Adenomyoepithelioma |
2 |
P63 (+); CALPONINE (+) |
|
Myoid hamartoma |
2 |
||
Filodes Tumor |
Low Grade |
26 |
|
Borderline |
1 |
||
High Grade |
12 |
||
Sarcomas |
Spindle cell |
2 |
|
Cystosarcoma |
1 |
AML (+): Vimentin: NEG; Keratin:NEG; Protein S100: NEG, desmin:NEG; CD31:NEG; CD34: NEG. |
|
Leiomyosarcoma |
1 |
Desmin (+); AML (+); PS100:NEG; Keratin:NEG; p63:NEG; Myogenin: NEG. |
|
Fibrosarcoma |
1 |
Desmin (+); KI67 (+), AML (+); Keratin:NEG; S100:NEG; CD34:NEG. |
|
Liposarcoma |
1 |
||
Carcinosarcoma |
1 |
||
|
Semi-differentiated angiosarcoma |
1 |
|
Table 3 Histological subtypes
The surgical treatment performed in these patients was 29 tumors, 15 simple mastectomies by tumor size, 3 adenomastectomies, 2 radiosurgical biopsies, 3 Madden operations, 1 margin enlargement (benign TF), 1 axillary formation resection.
Axillary emptying was indicated in 5 patients in the study group due to clinical and/or imaging nodal involvement. Of these, 1 corresponded to a high-grade phyllodes tumor, 1 to a low-grade phyllodes tumor, and 3 to sarcomas. In this group of patients, there was no axillary involvement in the anatomopathological study of the surgical piece. Only one patient diagnosed with malignant phyllodes tumor at 24 months after treatment had local and distant relapse.
When evaluating the recurrence rate in patients initially treated with radical surgery versus conservative surgery, it was observed that of 47 patients operated with a diagnosis of phyllodes tumor and sarcoma, 8 had local relapse. 50% of the recurrences were after radical surgery, while the remaining 50% were evidenced after conservative surgery (p=0.22). When subgroup analysis is performed, 3 high-grade TF, 3 low-grade TF, 1 borderline TF, 1 liposarcoma.
When comparing the rate of metastasis between the group with or without armpit treatment, distant disease is observed in 6.89% of the total population, corresponding to 5.16% of patients without lymphadenectomy and 1.72% of patients with axillary emptying.
Distant disease was detected in 4 patients, of whom 2 presented polymetastatic involvement and the remaining 2 oligometastatic involvement. Metastatic sites were bone, scalp, liver, and lung. Adjuvancy was performed for polymetastases with doxorubicin and for oligometathastics with adriamycin and cyclophosphamide. These metastases correspond to 3 malignant TF and 1 borderline TF, all as disease progression (Table 4).
Disease at a distance |
Injuries |
SLE |
Oligometastatic |
TF Malignant |
24 |
TF Malignant |
27 |
|
Polymetastatic |
TF Malignant |
24 |
|
TF Borderline |
9 |
Table 4 Distant disease with its SLE
Chemotherapy was indicated in 3 patients (5.17%), 2 corresponded to high-grade TF, and 1 to liposarcoma. All were performed after relapse.
Radiation therapy was indicated in 5 patients, 2 high-grade TF (1 post-lumpectomy, 1 simple post-mastectomy), 1 borderline TF (post-lumpectomy), 1 aggressive fibromatosis (post-lumpectomy), 1 mesenchymal cancer of spindle cells with atypia (stage IV).
When evaluating elements that could have an impact on the prognosis of malignant lesions, it is evident that the relapse rate was only increased in patients with tumor size > 5cm, with no difference observed in the analysis of the rest of the factors (Table 5).
Factors forecasts |
Relapse (N=8) |
p value* |
Age |
||
<40 |
25% |
0.45 |
>40 |
75% |
|
Tumor size |
||
<=5 cm |
37.50% |
1 |
> 5 cm |
62.50% |
|
Type of tumor |
||
Philodes Benigno |
37.50% |
0.63 |
Filodes Boderline |
12.50% |
|
Filodes malign |
37.50% |
|
Sarcomas |
12.50% |
|
Type of surgery |
||
Conservative |
50% |
0.64 |
Radical |
50% |
|
Table 5 Risk factors for relapse
Global survival measured in months had an average of 42.95, while disease-free survival was 31.71 months. The analysis of subgroups of patients according to age of disease presentation shows that early age is not a poor prognostic factor (SG<40 years: 45.15 months vs SG >40 years: 43.92 months).
In the comparative analysis of the different malignant lesions, we found in our sample that sarcomas have greater overall survival and disease-free survival than phyllodes tumors (Figure 2).
Fucocellular tumors are a heterogeneous group of lesions. Its frequency of presentation is low, which is why its treatment poses challenges.
In the population subject to this study, the mean age of presentation was 43.4 years, coinciding with the authors who reported higher casuistry in their reports such as McGowan, Zelek and Bousquet.3–5
The size of this type of tumor varies, ranging from 4 to 7cm. Ulceration and fixation to the chest wall are rare. Clinical suspicion of axillary nodal involvement is present in 20% of patients, with histological confirmation of such metastases being extremely rare. Distant metastatic disease has been reported in up to 20% of malignant tumors. In our sample, 49 patients presented unilateral, nodular and painless palpable lesions, 3 of them were associated with rapid growth and 5 with clinical axillary involvement, with local relapse in 8 patients in this group.2
In accordance with the available literature, we did not find in our population a characteristic mammographic or ultrasound image of this type of lesions.
Histologically, sarcoma is the most common subtype of tumors in this group and accounts for less than 0.04% of all breast cancers, however, in other literature reviews, phyllodes tumor is the most common. Our results reveal 8 patients diagnosed with sarcoma and 39 with phyllodes tumor. Of all patients diagnosed with phyllodes tumor, 66.67% were low-grade TF, 2.56% borderline, and 30.77% high-grade TF, similar to that reported by the WHO classification of 2012 (benign 60%–75%, borderline 15%–16%, and malignant 10%–30%).
The risk of recurrence in patients treated with conservative surgery vs mastectomy in our population is similar to that reported by Ganesh who analyzed 70 patients, showing similar proportions of relapses in both groups. Large excisions may be the treatment of choice for appropriate local control, and new excisions or mastectomies are reserved for cases where no negative margins are achieved with initial excision.6
Axillary dissection is not recommended routinely because lymph node involvement is infrequent, being performed in less than 1% of patients as in our population.2
Several reports in the literature have suggested that there are factors that predict the risk of distant recurrence after primary surgery, including tumor size. In our sample, the mean tumor size is 6.41cm, which is consistent with previous work, showing that tumors >5cm had a greater risk of relapse than smaller tumors. The low number of patients prevents multivariate analysis, due to the need for a certain number of events per variable for the statistical model to be stable, which is a limitation of this work.5–8
When a correlation is made between surgical margins and local recurrence, some authors demonstrate that compromised or close margins are an important risk factor for local recurrence. The NCCN recommends a free margin of at least 1cm, but authors such as Lenhard et al. found no significant differences in the surgical margin of those who developed a local recurrence compared to those who remained free of recurrence. It was possible to obtain from our sample, that all the patients had free margins and only one patient required enlargement of them. One of the limitations of our work is that the anatomopathological results extracted from the medical records did not report the surgical margins measured in unit of measure.6,9
In the analysis of subgroups of the analyzed population, the early age of presentation of the disease does not constitute a bad prognostic factor. When comparing the data with the literature, the publication of Sanjay et al reports that age >=40years had better SLE and SG, with no statistically significant difference. This finding differs from another study, where univariate analysis shows that the age group < =40years has better SLE and SG, but in multivariate analysis, age was not an independent factor affecting survival.10
Greater SG and SLE were observed for sarcomas, not consistent with the literature. This could be due to chance given in samples with small number of patients with this diagnosis. More cases are needed to obtain statistically significant conclusions.3,4,11–19
Fucocellular tumors are a very rare entity, which poses difficulties in both diagnosis and treatment.
The available literature is limited and the published studies have a low number of cases, being difficult to obtain definitive conclusions and extrapolable to this group of lesions.
There is currently no standardized treatment for this spectrum of entities. Broad excision is considered the treatment of choice for appropriate local control, and new excisions or mastectomies may provide additional protection in the event that no negative margins are achieved with initial excision.
The evolution of these patients is mainly related to the tumor size of onset, being observed as a factor of poor prognosis the lesions greater than 5cm.
It is considered of vital importance to have a greater number of publications that increase the casuistry, in order to provide information that reinforces the competences of professionals, with the aim of improving the prognosis of our patients.
None.
None.
The authors declare that they have no conflicts of interest.

©2023 Abate, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.