eISSN: 2377-4304


Research Article Volume 10 Issue 6
1Graduate Program in Psychology; Undergraduate course in Psychology, Institute of Psychology; Laboratório de Estudos, Pesquisa e Intervenção em Desenvolvimento e Saúde (LEPIDS), Maternity School Hospital, Universidade Federal do Rio de Janeiro (UFRJ), Brazil
2Institute of Psychology, Laboratório de Estudos, Pesquisa e Intervenção em Desenvolvimento e Saúde (LEPIDS), Maternity School Hospital, Universidade Federal do Rio de Janeiro (UFRJ), Brazil
3Department of Anesthesiology; Laboratório de Estudos, Pesquisa e Intervenção em Desenvolvimento e Saúde (LEPIDS), Maternity School Hospital, Universidade Federal do Rio de Janeiro (UFRJ), Brazil
4Laboratório de Estudos, Pesquisa e Intervenção em Desenvolvimento e Saúde (LEPIDS), Maternity School Hospital; Universidade Federal do Rio de Janeiro (UFRJ); Instituto de Medicina Social da Universidade do Estado do Rio de Janeiro (IMS/UERJ), Brazil
Correspondence: Ana Cristina Barros da Cunha, Graduate Program in Psychology; Undergraduate course in Psychology, Institute of Psychology; Laboratório de Estudos, Pesquisa e Intervenção em Desenvolvimento e Saúde (LEPIDS), Maternity School Hospital, Universidade Federal do Rio de Janeiro (UFRJ), Rua Ronald de Carvalho, 147/202, Copacabana, Rio de Janeiro, RJ, Brazil, Tel +55 21 981859182
Received: October 30, 2019 | Published: November 26, 2019
Citation: Cunha ACBD, Santana FVDS, Gribel GPDC, et al. Associations between a prior pregnancy loss and stress during the subsequent pregnancy and puerperal periods. bstet Gynecol Int J. 2019;10(6):414-417. DOI: 10.15406/ogij.2019.10.00476
Menotrophin is a protein-based hormonal therapy. It is used as a fertility medication that is given as injection either subcutaneously or intramuscularly. Menotrophin has not been previously reported to cause drug induced liver injury. Drug-induced liver injury (DILI) is commonly seen nowadays with the expansion of the drug industry it is associated with prescribed medications, over the counter drugs, herbal and dietary supplements. We report the first case of Menotrophin-induced autoimmune hepatitis in a 26years old Caucasian woman who was diagnosed with primary infertility due to failure to conceive after five years of marriage. She had received several cycles of Mentotrophin, and then developed new onset jaundice and fatigue associated with increase in transaminases. She had normal baseline liver function and enzymes prior to receiving treatment with Menotrophin. Evaluation showed no evidence of viral hepatitis, metabolic, alcoholic or vascular causes of liver injury. Autoimmune screening was positive for antinuclear antibody (ANA) with titer of 1:640 fine speckled; immunoglobulin G (IgG) level was 1900mg/dl. Antimitochondrial antibodies (AMA) and anti-smooth muscle antibodies were negative. Liver biopsy showed features of chronic hepatitis with interface hepatitis and prominence of plasma cells which best reflects autoimmune hepatitis. Her liver enzymes and bilirubin completely normalized after discontinuation of further Menotrophin therapy and starting treatment with prednisolone and Azathioprine.
DILI, Drug-Induced Liver Injury; ANA, Antinuclear Antibody; AMA, Antimitochondrial Antibodies; IgG, Immunoglobulin G; LH, Luteinizing Hormone; FSH, Follicle Stimulating Hormone Receptor; DIAH, Drug Induced Autoimmune Hepatitis; AIH, Autoimmune Hepatitis
Menotrophin is a female infertility gonadotropin treatment contains follicle stimulating hormone (FSH) and luteinizing hormone (LH) purified from the urine of postmenopausal women. It binds to the follicle stimulating hormone receptor (FSH), which results in ovulation in the absence of sufficient endogenous luteinizing hormone (LH). It also binds the LH receptor, thereby stimulating proper hormone release. Menotrophin is known to cause minor gastrointestinal symptoms such abdominal pain, nausea, vomiting and other unspecific symptoms. No other drugs or food interaction found.
We here present first case of drug induced autoimmune hepatitis (DIAIH) following treatment with Menotrophin requiring immunosuppressive therapy, that has hitherto not been described to best of our knowledge. The causality of autoimmune hepatitis (AIH) is uncertain but the disease can be triggered in some patients by external factors such as viruses or drugs. DIAIH can mimic many liver diseases, and its effect can range from a mild elevation of liver enzymes to liver failure.
Many drugs have been linked to cause AIH, which sometimes persist after drug discontinuation, suggesting that they awaken latent autoimmunity. Drug-Induced Autoimmune hepatitis (DIAIH) is still a poorly defined and an under-reported liver disorder. Histologically distinguishing DILI from AIH remains a challenge.
A 26years old Caucasian lady was diagnosed with primary infertility after failure to conceive despite ongoing attempts over five years. The patient received four cycles of treatment with Menotrophin within the last four years with approximate interval of 12months in between cycles. Each cycle consisted of five doses of Menotrophin given as intramuscular injection every other day. One month after the last cycle, the patient started to develop yellowish discoloration of sclera and skin associated with pruritus which was gradual and progressive with time. She also developed pale stool, and dark urine. There was No history of vomiting, nausea, abdominal pain, and change in bowel habit or weight loss. She had No history of alcohol consumption, and denied using recreational drug, other prescription or non-prescription drugs or herbal supplement.
On examination, vital signs were within normal limits. Despite being deeply jaundiced, she had normal level of consciousness and had no stigmata of chronic liver disease and negative abdominal finding. She had baseline investigations prior to starting Menotrophin that showed normal liver enzymes and liver synthetic functions. Her laboratory tests during this presentation showed Aspartate aminotransferase 590U/L, Alanine aminotransferase 504U/L, Total Bilirubin17.81mg/dl, Gamma-glutamyl transferase 486U/L, Direct Bilirubin 13.16mg/dl, albumin 2.4g/dl, alkaline phosphatase 366U/L, International normalized ratio 1.3, Hemoglobin 11g/d, total white blood cells 9.25x10Λ9/L, platelets count 188 x10Λ9/L. Further workup revealed immunoglobulin G (IgG) level 1900, B2 glycoprotein (IgG) 42.4 EU/ml, ANA 1:640 fines speckled.
AMA, anti-smooth muscle antibodies (ASMA), B2 glycoprotein (IgM), proteinase 3 antibodies (PR3), liver Kidney Microsomal antibodies (LKM), hepatitis B and C serology were all negative. Liver ultrasonography showed coarse echotexture with lobulated surface and enlarged caudate lobe, no portal vein thrombosis, normal biliary, no stones.
Liver biopsy revealed portal fibrosis with expansion and formation of portal-portal and portal-central bridging fibrosis. Marked mostly lymphocytic infiltrate containing plasma cells and occasional polymorphs & esosinophils were seen in portal tract, extending focally into the lobules with moderate interface & lobular hepatitis. No obvious hepatocyte rosette formation, though a few necrotic hepatocytes were present. The bile duct revealed focal injury through infiltration by polymorphs and lymphoid cells. The features of liver biopsy suggested chronic active hepatitis with interface hepatitis and prominence of plasma cells stage 3 (Figures 1-3).
The diagnosis of autoimmune hepatitis was based on Simplified diagnostic criteria of International Autoimmune hepatitis Group with a score of 7 points, which make her case fall in category of definite autoimmune hepatitis. 1 After establishing the diagnosis of AIH, she was started on prednisolone and Azathioprine. Her liver enzymes and synthetic function improved with time and then normalized after 4 months of treatment (Table1). Her prednisolone was tapered down until complete discontinuation and maintained on Azathioprine monotherapy.
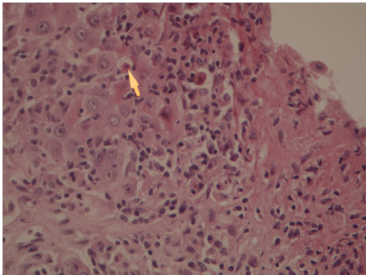
Figure 1 Liver biopsy showing Interface hepatitis revealing spillover of inflammation into adjacent liver parenchyma and necrotic hepatocytes. HE 10x20.
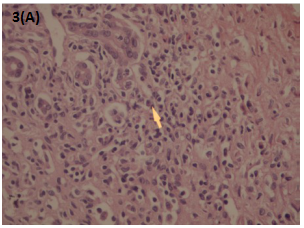
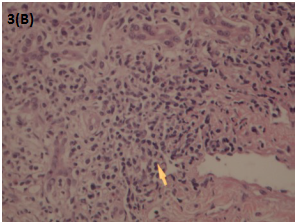
Figure 3A & B Liver biopsy Showing moderate to marked portal tract mostly lymphoplasmacytic inflammatory cell infiltrate. HE: 10x20
AST |
ALT |
ALP |
Total bilirubin |
|
Reference range |
0 -37U/L |
0 -45U/L |
46 -116U/L |
4 -21mmol/L |
1st day of treatment |
593 |
482 |
343 |
57.3 |
5th day of treatment |
278 |
286 |
318 |
47.47 |
15th day of treatment |
62 |
103 |
226 |
30.6 |
21th day of treatment |
36.3 |
45 |
176 |
22.1 |
Table 1 The table is showing liver enzymes improved with the treatment
Infertility has psychologic, emotional and financial consequences on families. The prevalence of primary infertility in the united states in married women is between 15-44years is 6%.2 However, with medical development, advanced fertility treatment options are more numerous and variable than ever.
Menotrophin is a female infertility gonadotropin treatment which may induce minor gastrointestinal adverse effects such as abdominal pain, nausea, vomiting and unspecific symptoms. DILI is a common cause of liver disease that could be associated with prescribed medications, over the counter drugs, herbal and/or dietary supplements. Its manifestations can mimic many liver diseases, and its effect can range from a mild elevation of liver enzymes to liver failure. DILI accounts for 10% to 50% of elevated liver enzymes in adults, and 10% of all acute hepatitis cases.3 Risk factors include (a) age; adults are at higher risk than children except for of valproic acid-induced DILI, which is more common in children; (b) women are at higher risk than men; (c) Alcohol abuse; (d) malnutrition.4 Few DILI cases may present with typical features of autoimmune hepatitis, which makes differentiating between the two challenging. DIAIH is still a poorly defined and an under-reported liver disorder. It can commonly be caused by minocycline, alpha-methyldopa, nitrofurantoin, hydralazine, and statins.5
Diagnosis of AIH could be challenging and therefore several scoring systems have been developed to standardize the diagnosis. The Simplified diagnostic criteria of International Autoimmune hepatitis Group Score showed 81% sensitivity and 99% specificity when using a cutoff score ≥7 points as a definitive diagnosis of AIH.1
In this case we present here, our patient scored 7 points; one point for positive ANA, two points for positive IgG, two points for liver histology & two point for absence of viral hepatitis; which qualify as definitive diagnosis of AIH.
DILI is still a diagnosis of exclusion especially with the lack of gold standard test or tool to establish the causality. The RUCAM (Roussel Uclaf Causality Assessment Method) or its previous synonym CIOMS (Council for International Organizations of Medical Sciences) score is a tool that could provide help in guiding a systematic and objective evaluation of patients suspected to have DILI.6,7 RUCAM score ranges from -9 to +10 with higher score indicates higher likelihood of DILI.6 The diagnosis of DILI in our patient is classified as probable based on RUCAM score of +6.
It is important to distinguish drugs as triggers of a self-perpetuating autoimmune liver disease from immune-mediated drug-induced liver injury (IM-DILI). Immune-mediated DILI nearly always resolves or becomes quiescent when drugs are withdrawn. Another possibility is that AIH was quiescent and remains undiagnosed until a drug triggered a new autoimmune process. Thus, to attempt to make a proper diagnosis of the type of immune process affecting the liver is challenging.5
In addition, Recognition and discontinuation of the drug causing DIAIH is the most crucial step in management, along with evaluating the severity and the characteristics of injury. Liver biopsy is not essential for diagnosis. The primary treatment is with corticosteroid therapy.
Clinical pictures and histopathological features of DILI and AIH are similar, but the key point of differentiation between the two is the response after cessation of immunosuppressive therapy. Successful cessation has been reported in DIAIH while relapses has been reported in AIH.8
This report describes a case of probable drug induced autoimmune hepatitis following treatment with Menotrophin for infertility in a young female with no previous known liver disease and with documented normal liver enzymes prior to therapy. We suggest checking liver function test prior to staring and during such treatment since there is a probability of serious DILI associated with its use.
None.
Author declares their no conflicts of interest.

©2019 Cunha, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.