eISSN: 2377-4304


Case Report Volume 12 Issue 3
1Associate Professor, Department of Pathology, Dr. Ram Manohar Lohia Institute of Medical Sciences, India
2Assistant Professor JNMC AMU Aligarh, India
3Retired Professort, Department of Pathology, University College of Medical Sciences and Guru Teg Bahadur Hospital, India
4Professort, Department of Pathology, University College of Medical Sciences and Guru Teg Bahadur Hospital, India
Correspondence: Dr. Ruquiya Afrose, MD, Assistant Professor, Department of Pathology, JNMC, AMU, Aligarh, India, Tel 9219716166
Received: June 01, 2021 | Published: June 21, 2021
Citation: Malhotra KP, Afrose R, Agarwal S, et al. Adult granulosa cell tumor with Mucinous cystadenoma of ovary: a unique case with insight into histogenesis. Obstet Gynecol Int J. 2021;12(3):190-193. DOI: 10.15406/ogij.2021.12.00574
Mixed ovarian tumors are of common occurrence. In this category are placed rare novelties displaying composite granulosa cell and mucinous tumor elements. Such a combination of stromal and epithelial elements may be a chance association of two discrete tumors. Intriguing still is the intimate admixture of these two elements which has been reported only in two cases till date. (1,2) We report the third such case and review the previous cases with an effort to elucidate their enigmatic histogenesis.
Keywords: concurrent tumors, granulosa cell tumor, histogenesis, mucinous cystadenoma, ovary
Mucinous elements are a common accompaniment in several mixed ovarian tumors, frequently so in mixed epithelial tumors. The association of mucinous elements has also been noted along with sex cord stromal tumors, a sizeable proportion admixed with Sertoli Leydig cell elements. The combination of mucinous elements with an adult granulosa cell tumor has been reported till date in four previous cases, one of which was a mucinous cystadenocarcinoma.1–4 We report a similar case along with a review of the previously reported cases and discuss the existing theories regarding their histogenesis.
A seventy five year old post menopausal female presented with vaginal bleeding for six months. She was menopausal for the past twenty five years. No other relevant family or personal history was elicited. She had never been on hormone replacement therapy. General examination revealed mild pallor. No mass was palpable per abdomen. On examination per vaginum a 4x4cm mass was palpated in the left pouch of Douglas.
Transvaginal ultrasonography was suggestive of a multi-cystic left adnexal mass measuring 5.5x5.3cm. Transabdominal ultrasound revealed a heterogenous predominantly cystic left ovarian mass measuring 5.5x4.5cm. There was no ascites. Serum CA125 was 41.58u/ml and Inhibin was raised at 83.3pg/ml. A clinical diagnosis of benign cystic tumor in the ovary was considered with unexplained elevation of inhibin levels. Staging laprotomy with total abdominal hysterectomy, bilateral salpingo-oophorectomy, infracolic omentectomy and pelvic lymph node sampling was performed. Postoperative course was uneventful. The patient is currently on follow up and well five months after surgery.
Pathological features
The left ovary measured 5.5cm in diameter and had a smooth encapsulated surface. The cut surface revealed multiple tiny cysts with intervening solid yellowish areas. The cysts were filled with mucoid fluid. No hemorrhagic or necrotic areas were identified. Uterus, cervix and bilateral fallopian tubes were unremarkable. The endometrial cavity was slit like, endometrial thickness being 0.7cm. The right ovary was enlarged by a serous fluid filled cyst measuring 1cm in diameter.
Microscopic sections from left ovary revealed variable sized cysts lined by single layered tall columnar mucin secreting epithelium of endocervical type. The epithelial cells stained positive with mucicarmine. Goblet cells were not identified. The epithelium did not divulge any evidence of atypia, increased mitosis or multilayering. Intimately admixed with the benign mucinous component were diffuse sheets of plump to ovoid cells with uniform round to oval pale nuclei, many with longitudinal nuclear grooves and indistinct cytoplasm reminiscent of granulosa cells. (Figure 1) Focal thecomatous areas and Call Exner bodies were seen.
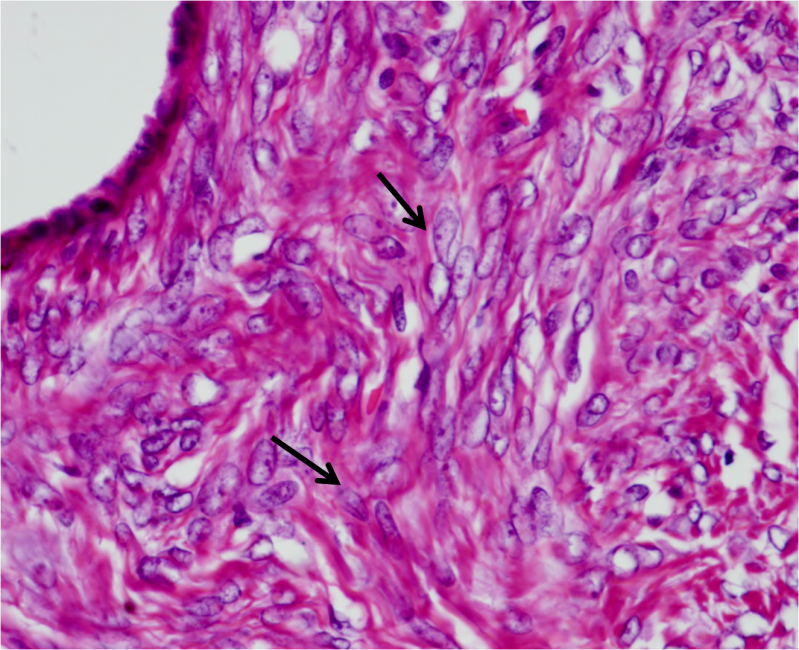
Figure 1 Granulosa cells with ovoid nuclei and occasional nuclear grooves (arrows). Columnar mucinous epithelium in left upper corner.
Immunohistochemical staining showed pan cytokeratin and epithelial membrane antigen positivity in the mucinous epithelium. The granulosa cells were positive for Vimentin, Inhibin and Calretinin. (Figure 2) The endometrium revealed simple hyperplasia and right ovary showed a follicular cyst. Cervix and tubes were unremarkable. The pelvic lymph node showed reactive hyperplasia.

Figure 2 Immunohistochemical profile of the tumor. A- Epithelial Membrane Antigen positivity in mucinous epithelium and B-Vimentin, C- Inhibin and D- Calretinin positivity in granulosa cells.
A diagnosis of adult granulosa cell tumor with heterologous mucinous cystadenoma elements was rendered based on morphology and immunohistochemical findings. Hyperestrogenic features including postmenopausal bleeding, endometrial hyperplasia and simple cyst in opposite ovary and raised Inhibin were supportive of an estrogen secreting granulosa cell tumor element.
Mixed ovarian tumors with both elements of Mullerian derivation one of which is an endocervical type of mucinous cystadenoma is of common occurrence and explained by the fact that tumor cells differentiate towards two closely related epithelial structures.1,5 Mucinous tumors found in association with teratomas are usually intestinal in type, of germ cell derivation and probably represent overgrowth of the mucinous epithelium. A report of a concurrent granulosa cell tumor, mucinous cystadenoma and cystic teratoma in the same ovary exists.6 These were considered to represent secondary tumors arising from foci of sex cord stromal and epithelial cell differentiation in a cystic teratoma.
The histogenesis is difficult to explain when an epithelial tumor coexists with another tumor of sex cord stromal origin outside the setting of a teratoma. Upto 18% of cases of sertoli leydig cell tumors have been found to contain mucinous cystadenomatous elements.7 A stromal tumor with minor sex cord elements has also been reported in association with a mucinous cystadenoma.8 It has been hypothesized that the mucinous elements are of heterologous metaplastic origin, supported by the fact that the two elements were closely intermingled and showed zones of transition from one cell type to another.3
From the literature available to us, four cases of mucinous cystadenoma and two of mucinous cystadenocarcinoma concurrent with adult granulosa cell tumors could be identified. Their salient features are presented in Table 1. The clinical features were akin to granulosa cell tumors rather than mucinous cystadenomas including presentation at postmenopausal age groups with vaginal bleeding. Similarly endometrial hyperplasia and polyp could be explained on the basis of hyperestrogenic effect of the granulosa cell element. Two of the cases reported showed separate areas of mucinous epithelium and granulosa cell component without intermingling of the two. It is likely that these resulted from the concurrent occurrence of two varied tumors which is not unexpected by chance.
|
Case report |
Price et al |
Chandran et al |
Doussia A et al |
McKenna et al |
Kushida Y et al |
Staats PN et al |
Subrahmanya NB et al |
Present case |
|
Age (years) |
63 |
83 |
49 |
57 |
76 |
73 |
50 |
75 |
|
Presentation |
Lower abdominal discomfort, distension |
Post menopausal bleeding |
Menorrhagia |
Abd distension, vomiting, wt loss |
Lumbago |
Post menopausal bleeding |
Lower abdominal pain |
Post menopausal bleeding |
|
Parity |
3 |
10 |
1 |
N/A |
N/A |
N/A |
3 |
3 |
|
Inhibin level |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
83.3pg/ml |
|
CA 125 level |
WNR |
N/A |
N/A |
90 U/ml |
120 U/ml |
30 U/ml |
19.44 |
41.6 U/ml |
|
U/mL |
||||||||
|
Ovary gross |
Multiple cysts with thickened walls |
Solid-cystic |
Cyst + nodule |
Cyst + nodule |
Solid-cystic |
Multilocular cysts |
Multilocular cysts |
Multilocular cysts; intervening solid areas |
|
Ovarian size |
20x16x11cm |
10x10x7 |
4x2.5x1 |
30x28x20 |
10x7x7 |
22x14x14 |
8.5x5.5x4cm |
6x6x5 |
|
Mucinous epithelium |
Endocervical |
Not described |
Endocervical |
Intestinal |
Endocervical |
Intestinal |
Intestinal |
Endocervical |
|
Mucinous Neoplasm |
Benign |
Malignant |
Benign |
Benign |
Benign |
Borderline malignant |
Benign |
Benign |
|
Mitoses |
Nil |
++ |
Nil |
Upto 3/10hpf |
N/A |
N/A |
N/A |
Nil |
|
Leutinization |
Nil |
Scattered clumps |
Nil |
N/A |
N/A |
N/A |
Nil |
Nil |
|
Thecomatous stroma |
Nil |
Present |
Nil |
Nil |
Prominent |
Prominent |
Nil |
Nil |
|
IHC mucinous elements |
AE1/AE3,CAM 5.2, EMA,CEA |
CK,EMA, CEA |
N/A |
CK7, focal CK20 |
CK (pan, 7,18,19), CA125, S-100, focal CEA & EMA |
N/A |
CK 20,7 |
CK, EMA |
|
IHC granulosa component |
Vimentin |
Vimentin |
N/A |
Inhibin, calretinin |
Vimentin, CK (18,19,focal pan), focal Carletinin. Inhibin α negative |
N/A |
Focal Inhibin CD99; Calretinin |
Vimentin, Inhibin,calretinin |
|
Opposite ovary |
U/R |
Small, atrophic |
U/R |
U/R |
N/A |
Cystic |
N/A |
Cystic follicle |
|
Uterus |
No e/o hyperplasia in hysterectomy done 13 years prior to development of ovarian tumor |
Benign cystic hyperplasia |
Leiomyomata; endometrium U/R |
Endometrial polyp |
U/R |
Leiomyomata, adenomyosis, weakly proliferative |
Leiomyomata |
Simple hyperplasia |
|
Tubes,cervix |
U/R |
U/R |
U/R |
U/R |
U/R |
U/R |
U/R |
U/R |
|
Proposed histogenesis |
Heterologous |
Composite |
Composite |
Heterologous |
reactive stromal hyperplasia in a pre-existing mucinous neoplasm |
Thecomatous stroma of a mucinous neoplasm |
Heterologous |
Heterologous |
Table 1 Comparison of features of concurrent mucinous and granulosa cell tumors reported previously and the present case
N/A, data not available; WNR, within normal range; U/R, unremarkable; CK, Cytokeratin; EMA, epithelial membrane antigen; CEA, carcino embryonic antigen
Our case similar to three earlier cases describes intimate association of the two elements with endocervical type epithelium.1,9,10 Such intermingling cannot be explained by chance occurrence of the two neoplasms together. Two theories can be considered for this association- a likelihood that the mucinous elements represent heterologous metaplasia in a granulosa cell tumor and another of an origin of the granulosa cell tumor within the reactive stroma of a mucinous neoplasm. A heterologous metaplasia akin to sertoli leydig cell tumors concurrent with mucinous elements is unlikely since both intestinal and endocervical types of mucinous metaplasias have been reported. A point in favour is their association with endocervical type of epithelium which is of Mullerian derivation and likely to occur in an ovarian metaplasia, as opposed to intestinal epithelium which is foreign to the ovary.
Mc kenna et al reported close intermingling of granulosa cell elements with intestinal type of mucinous epithelium.4 This is similar to the cases of sertoli leydig cell tumors with intestinal mucinous epithelium.5 Further studies are warranted to explain the origin of such mixed tumors. The possibility of a teratomatous origin cannot be disregarded in such cases.
We report this rare case of heterologous benign mucinous metaplasia in a granulosa cell tumor. The recognition of such an entity aids in understanding its histogenesis and is a teaching point for Gynecologists and Pathologists whereby the hyperestrogenic features of a cystic tumor likely to be denoted a benign cystadenoma on radiology can be explained. The prognostic implications of this association are yet to be seen.
None.
None.
The authors declare no conflicts of interest in preparing this article.

©2021 Malhotra, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.