eISSN: 2377-4304


Objective: To report a case of malignant transformation of a mature teratoma and to review the available literature regarding its incidence of degeneration, histological types, clinical characteristics and therapeutic approach of this type of tumor.
Materials and methods: We present the case of a woman with malignant degeneration of a germ cell tumor of benign histology, attended at a fourth level institution of complexity, located in Guadalajara, Mexico. A review of the literature published in the Medline databases via PubMed, Embase and SciELO was performed using the search terms: "Germ cell tumor", "mature teratoma", " squamous cell carcinoma ". The search was limited by language (articles in English and Spanish).
Results: We retrieved 21 references that met the inclusion and exclusion criteria. Mature teratoma is composed of tissues from all layers of germ cells, more common in postmenopausal women, between the fifth and sixth decades of life, their diagnosis should be suspected in the presence of established criteria, surgical treatment continues being the cornerstone in the management being able to add cytotoxic agents based on platinos as adjuvant.
Conclusion: The probability of malignant degeneration of a mature teratoma is extremely rare, the most common histological type is squamous cell, studies are required to evaluate the impact of chemotherapeutic agents on adjuvant therapy and the use of radiotherapy.
Keywords: Germ cell tumor, teratoma, dermoid cyst, squamous cell carcinoma
Mature Cystic Teratomas are the most common ovarian germ cell tumors and the main neoplasia in women younger than 20 years, accounting for approximately 20% to 25% of benign lesions1 and appear bilaterally in 10 to 17% of the cases. The incidence is estimated from 1.2 to 14.2 cases per 100,000 people per year.2 They arise from the primordial germ cells, so they can consist of multiple types derived from one or more of the 3 germ layers (ectoderm, mesoderm, endoderm).
TQM usually remain asymptomatic or cause minimal symptoms. The most common signs and symptoms include increased abdominal circumference, abdominal pain and/or bloating, and palpable abdominal or pelvic mass. If there is invasion to adjacent structures, gastrointestinal or urinary symptoms may occur.3 Rarely manifests with menorrhagia, fever or cachexia.
The most frequent associated complications are torsion (6%), malignant degeneration (0.17 to 3%),4 ovarian rupture (1 to 2%) and infection (1%). One of the theories for malignant degeneration is based on the long-term presence of a mature teratoma in the pelvic cavity exposed to different hormonal, environmental and potential carcinogenic factors.5,6
During their transformation, malignant components can evolve from any of the three germ layers, resulting in different types of histological tumors. More than 80% are squamous cell carcinomas (derived from the ectoderm), cutaneous tumors (basal cell carcinomas, sebaceous tumors, malignant melanomas, neuroendodermal tumors).7,8
In relation to the histogenesis of epidermoid carcinoma originating in mature teratoma, the association with in situ squamous dysplasia and carcinoma is attributed to the epidermis of the epidermis that delimits the dermoid cyst or mature teratoma.9
Most mature cystic teratomas are detected 15-20 years before undergoing secondary malignant transformation.10
The diagnosis of malignant transformation of TQM poses difficulties for physicians and it is necessary to suspect the presence of a tumor greater than 10 cm in a post-menopausal patient (between the 5th and 6th decade of life), with adhesions and in many cases rupture of the capsule. These tumors show to a great extent the presence of nodular, papilliferous or cauliflower growths that protrude into the cyst cavities.10
Serum tumor markers such as squamous cell carcinoma (SCC) antigen, CA-125, CA19-9, and carcinoembryonic antigen (CEA) are useful in distinguishing mature cystic teratoma from malignant transformation. Tissue polypeptide antigen and macrophage colony stimulating factor may also help predict malignant transformation in this tumor.11
Removal of the entire tumor, according to onco-surgical treatment principles, is essential (complete cytoreduction). There is evidence that cisplatin is active against gynecological SCC and is the most studied among alkylating agents. Radiotherapy may lead to increased morbidity and is recommended in pelvic-limited disease.12,13
A 59-year-old patient with a clinical manifestation who started in March 2016, consisted of alterations in bowel habits (constipation), which is why she attends a doctor who requests imaging studies demonstrating a complex ovarian cyst. She is submitted to exploratory laparotomy by performing left salpingo-oophorectomy and Histopathological findings are sent to our institution.
As antecedents of importance, he referred to the death of his father due to lung cancer and the performance of three cesarean sections, allergic to penicillin and sulfas, menarche at age 13, onset of sexual life at age 30, number of sexual partners 1, formula obstetric: G3 P3 A1 C3, date of last menstruation at age 47, use of oral contraceptives (OCs) for one year.
Physical examination showed ECOG: 1, Karnofsky: 90, without hemodynamic instability. Abdomen: Semiglobular per adipose panicle, surgical scar in good condition, slightly painful to superficial palpation, without data of peritoneal irritation.
Speculum: Atrophic cervix apparently healthy, no visible tumor, cytology is taken, bimanual examination by recent surgery is not possible.
Report of pathology; invasive epidermoid carcinoma, moderately differentiated, keratinizing, originating in a mature cystic teratoma monodermic ovary. The neoplasia presents affectation and focal capsular rupture, with lymphovascular permeation, without perineural invasion. The diameters of the lesion are 10.1 x 99.9cm (Figures 1–3). Salpinge congestive and edematous without evidence of infiltration by neoplasia.
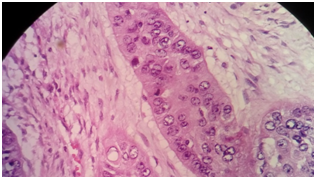
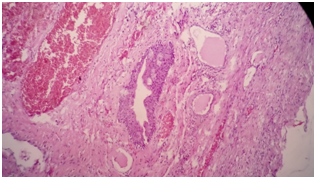
We requested revision of laminates and paraffin blocks by our institution confirming finding. CA-125 of 47.3U/ml, is taken to surgery to complete staging achieving optimal cytoreduction, and staged as IC3.
Receives 3 cycles of chemotherapy with cisplatin, etoposide, and bleomycin. During surveillance, CA-125 was elevated to 71.4U/ml, deep vein thrombosis of the left lower limb, abdominal computed tomography (CT) with left hydronephrosis and pelvic mass in the obturator fossa with vascular and nerve involvement. Palliative QT is initiated with the patient's subsequent death in April 2017 secondary to pulmonary thromboembolism.
A literature search was conducted using Medline databases via PubMed, Embase and SciELO, from January 2013 to August 2017. The keywords used were "Germ cell tumor," "teratoma," "dermoid cyst," and "Squamous cell carcinoma", in English or Spanish. We excluded studies for which the full text was not available. It was also supported by a guiding text.
Ethical aspects. Authorization was requested for the publication of the clinical case. The necessary precautions were taken to guarantee the confidentiality of the information and the anonymity of the patient.
Of the articles reviewed in the different electronic databases, 20 references met in full with the inclusion criteria. All were case reports or series. We analyzed 15 of them, two of which contained two cases and two contained four cases, for a total of 23 patients with malignant transformation of a mature cystic teratoma.
The age of presentation fluctuated between 30 and 69 years with an average of 52 years, an average corresponding to that described in the literature.14,15
Regarding the symptomatology that motivates the search for medical assessment, abdominal pain, which is present in 15 patients, is predominantly and significantly followed by incidental findings, abdominal distension, and gastrointestinal and urinary symptoms.
The most widely used imaging study for assessment of adnexal mass was pelvic ultrasound in 16 patients, followed by abdomino-pelvic CT in 14 patients and finally nuclear magnetic resonance in 6 patients.
The most commonly used tumor marker was the CA-125, 19 patient, with a 42.1% of elevated results for the estimated normal range, the second was the CA-19.9, 11 patients, with 63.6% of elevated results, the following were; Ag-SCC in a total of 8 women and CEA in 6 women, with altered results in 62% and 50% respectively. AFP and B-HCG levels were measured in 5 patients and none were found to be altered. Although no significant correlation has been described between the levels of these tumor markers and the FIGO clinical stage, evidence of elevated levels would be predictive of poor survival prognosis.16
The most frequently performed surgical procedure according to the analysis was unilateral salpingo-oophorectomy by laparotomy, followed by staging/cytoreductive surgery, hysterectomy with bilateral salpingo-oophorectomy by laparotomy accompanied in some occasions by omentectomy. Although there is no consensus, the surgical treatment chosen is generally the same as that offered to patients with ovarian epithelial cancer, cytoreduction, looking for the macroscopic absence of the disease and to be able to perform it at first intention we must orient the pathologist so that their search is directed towards this entity during the evaluation of the freezing study.17,18
Although it was not possible to calculate the average volume of adnexal tumors, due to lack of data, the largest diameter was taken and an average of 16.85 cm was calculated, corresponding to that found in the literature that would be greater than 10cm.
Of the 23 cases reported, 33.3%, ie 7 patients, presented rupture of the tumor as a finding at the time of surgery, two analyzes did not provide information on this. 55% of the tumors were reported from the left ovary and 45% from the right ovary.
A total of 3 peritoneal washes were positive for malignant cells, being 27.2%,10,15,16 reported as negative of 72.7%, and a total of 12 cases did not or did not report it.
Eighty-two percent (19 patients) presented squamous cell carcinoma as the malignant transformation of mature cystic teratoma. The other cases were mucinous adenocarcinoma, malignant melanoma,19 papillary thyroid carcinoma, and sebaceous adenocarcinoma.20 7 patients were submitted to surgery after the definitive study of pathology; complete hysterectomy and salpingo-oophorectomy were performed in all of them, pelvic and para-aortic lymphadenectomy was performed in 5 cases, omentectomy in 3 cases and an appendectomy. One of the surgeries was defined as "debulking" without specifying each procedure.21
Fifteen clinical stages were also reported according to the FIGO classification; 46.6% corresponded to a stage I, 13.3% to a stage II and 40% to a stage III. 14 patients reportedly received adjuvant chemotherapy. The predominant cytotoxic agent was platinum derivatives, in 11 cases (78.5%), of which 50% was cisplatin and 50% for carboplatin, (a report did not specify the type of platinum). Paclitaxel was used in 50% of patients, bleomycin in 21.4%. They received vincristine two patients, mitomycin C two patients, etoposide and 5FU in one case respectively. In no case is the use of white therapy documented.
In 5 patients recurrence was documented between 3 and 4 months after surgery. Platinum was used as the first choice in three cases (2.16), associated or not with paclitaxel, docetaxel, ifosfamide or gemcitabine.
Overall survival, regardless of FIGO clinical stage, and calculated to date of reports or death of patients, was estimated at an average of 34.5 months in general. A higher average than that evidenced in other analyzes where it was less than 12 months.19
The malignant transformation of a malignant cystic teratoma is a rare entity and its diagnosis should be suspected in the presence of rapidly growing tumors in patients within the fifth and sixth decade of life and with imaging studies that orient us towards a dermoid cyst. Abnormal elevation of the antigen for squamous cell carcinoma may lead to suspicion of the entity, as well as elevation of CA-125, CA-19.9 and CAE. Surgery will always be the mainstay for treatment associated with the use of platinum-based cytotoxic agents. Studies with single or concomitant radiotherapy with chemotherapy should be performed in addition to the use of agents for white therapy.
To the doctors, Guillermo E. Juárez López and Claudia B Castro Ortega, anatomopathologists, for the photographs provided for the present publication.
The authors declare no conflicts of interest

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.