eISSN: 2377-4304


Objectives: The cellular immune system mediated by cytotoxic T lymphocytes (CTLs) and Natural killer (NK) cells are thought to play an important role in the ultimate decline of Squamous Intraepithelial Lesions (SILs). In this study, Natural killer and granzyme B (GranzB) positive T–cells were quantitatively evaluated in cervical tissue–samples obtained from women exhibiting invasive cancer (n=5) and progressive grades of Cervical Intraepithelial, (CIN)I (n=5), CINII (n=5) and CINIII (n=5), in addition to five normal controls.
Methods: The precise number of cervical T–cells subpopulations, positive for the cytotoxic marker GranzB was determined by performing a morphometric analysis of positive cells detected by Immunohistochemistry, (i) in the entire area of lesioned epithelium from CIN samples and, (ii) within the invasive epithelial areas and in zones of surrounding lymphocyte infiltration in cervical cancer tissues.
Results: In normal cervix, a lower density of GranzB+ cells was observed, in comparison with the number of those cells detected in pre–neoplastic and cervical cancer tissues. Large granzyme B positive cells, exhibiting morphological aspects of Natural killer cells (NK–like cells) could be easily observed within the lesioned epithelium in CIN samples, as well as within the tumor nests and in the surrounding stroma, in invasive cervical cancer tissues. The number of GranzB+–cytotoxic T lymphocytes exceeded the number of NK–like cells in both, CIN tissues and invasive cancer. Increased densities of GranzB+ cells were observed in CIN samples, with an important prevalence of these cytotoxic cells in CINIII samples.
Conclusion: Our results indicate that the morphometric evaluation of GranzB+ cells’ number is a quantitative and effective approach to determine the number of NK–like cells and cytotoxic T lymphocytes in cervical tissues. Our findings also suggest that these immune–cells are possibly involved in the cervix surveillance against the cervical lesion development.
Keywords: natural killer cells, T–lymphocytes subpopulations, immunohistochemistry, SILs, cervical intraepithelial neoplasia (CIN), cervical cancer, morphometric analysis
Cervical cancer is the second most prevalent gynecological malignancy in women worldwide.1 Although near 99% of cervical cancers are caused by a persistent infection with high-risk human papillomavirus (HPV),2,3 this infection is not exclusively sufficient for the development of most squamous cancer of the cervix and their precursor lesions.4 According to the Bethesda Classification System, low-grade squamous intraepithelial lesions (LSILs) correspond to light dysplasia/CINI, and cell changes associated with HPV, whilst high-grade squamous intraepithelial lesions (HSILs) correspond to moderate dysplasia/CINII and to severe dysplasia/Ca in situ/CINIII.5-7 HPV infection is normally controlled by the host immune response, and the vast majority of HPV-infected women clear the virus within two years.8 However, failure to mount an effective immune response is related to the inefficient activation and priming of the innate and adaptive immune responses of the host that facilitates viral persistence.9 It still remains unclear which immune cells are implicated in the virus eradication process. Natural killer (NK) and cytotoxic T lymphocyte (CTL) can play an important role in the elimination of virus-infected and tumor cells.10 Classically, NK cells are defined as a CD3-CD16+CD56+ lymphocyte subpopulation which are mainly found in the peripheral blood but can be also present in tissues, such as those from the uterine mucosa.11 Cytotoxic activity of NK cells is mediated by exocytosis of preformed granules containing perforin and granzymes.12 Despite the pivotal role of cytotoxic immune cells such as Natural killer in the host resistance to viruses and tumors, evaluation of the NK densities directly from cervical pre-neoplastic and neoplastic tissues has been only investigated in relatively few studies.13-18 Therefore, the aim of this study was to perform a quantitative analysis of NK cells and T-lymphocytes positive for GranzB directly from lesioned areas of cervical tissues with SILs and invasive cancer. Since we aimed to evaluate the precise number of cytotoxic cells in progressive stages of cervical lesions, our study analyzed pre-neoplastic samples categorized as CINI, II and III lesions.
Tissue samples
Twenty cervical samples obtained by LEEP (Loop Electrical Excision Procedure) or colposcopy-directed biopsies were retrospectively obtained from an archive of Paraffin-Embedded Tissues (PETs), from a large histopathological diagnostic routine service Laboratory. Histopathological diagnosis were independently reviewed and confirmed by three certified pathologists (including A.T. and P.A.F.), and corresponded to 5 CINI, 5 CINII and 5 CINIII cases, in addition to five cases of invasive cervical cancer. Five women who had undergone hysterectomy for benign conditions, and presented negative cytology and normal colposcopic/examination before hysterectomy were included as normal controls. This study was approved by the local Institutional Ethical Review Board.
Immunohistochemistry
Serial sections (5µM thick) were obtained from PET samples and one was stained by H&E to confirm the histopathological diagnosis. Consecutive sections were processed for IHC analysis. For antigen retrieval, sections were immersed in xylene to remove paraffin, re-hydrated and heated at 95°C in water-bath for 30 minutes (min) in Tris-Edta (pH 6.0). Blocking of nonspecific binding sites was performed with 2%BSA (Bovine Serum Albumin) in 1xPBS for 60min at room temperature (RT). After washing, sections were incubated at 4°C overnight with the anti-human primary granzyme B rabbit-polyclonal antibody (code Ab4059, Abcam, USA) diluted at 1:100 in 1xPBS-2%BSA). Blocking of endogenous peroxidase was performed with H2O2 at RT for 30 min. Sections were next incubated with secondary antibodies – Peroxidase Rabbit IgG (Vectastain ABC-PK6101 kit, Vector Laboratories-USA) for 30min, washed in 1xPBS and incubated with the ABC solution. Tissue-sections were counterstained with Harris’ hematoxylin, dehydrated and mounted in Enthelan (Merck/Millipore-Germany). Appropriate negative and positive controls were included in every batch of tests. Negative controls consisted of the primary antibodies omission, and they were invariably negative. Cells displaying cytoplasmic and membrane brown staining, in addition to appropriate morphological features were included. Large cells frequently exhibiting proeminent nuclei, cytoplasmic processes and strong labeling were considered as Natural killer-like cells as previously described,14 whereas rounded-shape smaller cells, displaying strong positivity for GranzB antibody were considered as cytotoxic CD8+T-cells.19 In normal tissues, the number of granzyme B positive cells was ascertained in representative areas of intraepithelial positive cells, while in CIN tissue-samples the GranzB+ cells’ number was evaluated in the whole intraepithelial-lesioned area. In SCC samples, the number of stromal positive cells was determined within the tumor nests, as well as in surrounding areas of epithelial invasion.
Morphometric analysis of granzyme B positive cells
The GranzB+ cells number was determined in each SIL sample in the area covering the whole intraepithelial lesion. Images obtained at high-magnification fields (400X) were digitalized, and the quantitative determination of positive GranzB cells was performed by using the software KS400 (Image Analyzer Carl Zeiss, Oberkochen, Germany). In normal tissues, GranzB+ cells number was ascertained from an extensive epithelial tissue-area which did not exhibit any cellular abnormalities. In SCC samples, quantification of GranzB positive cells was performed within the invasive epithelial areas, as well as in zones of surrounding lymphocyte infiltration. Positive cells were counted, and the surface areas of both epithelium (normal and CIN samples) and stroma (SCC tissues) were determined by the software KS400. The median values of positive cell number were expressed per mm2 of tissue areas.
Statistical analyses
Data were expressed as median, range, first and third quartiles. The Kruskal-Wallis (non-parametric) 1-way ANOVA test was used to determine significant differences among three and more groups. Statistical significance was considered as P<0.05.
Natural killer-like cells and Granz+ T-cells evaluation in cervical tissues
In normal cervix, NK-like cells positive for GranzB, exhibiting typical morphology and a large size were visualized within the normal epithelium, but mainly at the adjacent stroma lying the epithelium (Figure 1A). Natural killer-like cells were also present in the epithelium of CINI samples, where they exhibited a particular location at the lower third stratum (Figure 1B). NK-like cells present in CINII and CINIII tissues were preferentially placed at the middle epithelial layer in CINII samples (Figure 1C), and at the upper epithelium stratum in CINIII tissues (Figure 1D). In cancer tissues, NK cells were mainly observed within and surrounding the tumor nests (Figure 1E). Smaller and positive GranzB cells displaying strong labelling and a rounded-shape were also observed in normal epithelium (Figure 1A) and in CIN tissues (Figure 1B-D), where they exhibited a more variable location. These smaller and GranzB+ lymphocytes could be mainly observed at the entire epithelium thickness from CINII and CINIII tissue samples (Figure 1C-D), as well as within the cancer nests and surrounding stroma. Immunohistochemical analysis of these small and rounded-shape cells confirmed their identities as CD8+ T-cell lymphocytes (submitted manuscript). Differences in morphology and size which were detected between Natural killer-like cells and CD8+ T-cells can be observed in Figure 1F.
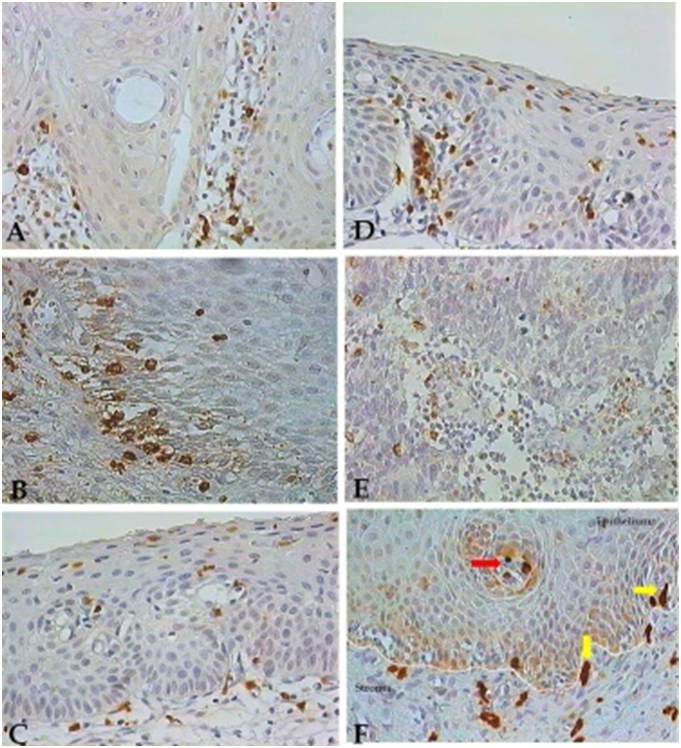
Figure 1 Natural Killer-like and granzymeB (GranzNatural Killer-like and granzymeB (GranzB) positive T-cells in cervical tissues. NK-like cells detected by IHC and exhibiting a brown color are shown in the cervical epithelium from normal (A) and CINI (B), CINII (C) and CINIII (D) tissues, and within the cancer nests (E) in the stroma of cervical tissues. Natural Killer-like cells exhibiting a typical large size (yellow arrows), and small GranzB-positive T-cells (red arrow) are shown within the epithelium and in the adjacent stroma of a CINI tissue sample (F). 400X magnification.
Quantification of granzyme B positive cells subpopulations
All numerical densities (expressed as median [25%-75%] cells number per mm²) of T-lymphocytes positive for granzyme B are shown in Table 1, as well as in Figure 2. Normal epithelium showed a low number of GranzB+ cells, when compared with those present in SIL lesions. Increased numbers of NK-like cells and of GranzB+ T-cells were observed in SIL tissues, but especially in the epithelium of CINIII samples. The densities of GranzB+ T-cells were notably higher than the number of NK-like cells when considering tissues from similar CIN grades (Figure 2 & Table 1). However, a relevant reduction in the number of both types of GranzB positive cells (NK-like cells and CD8+ T-cell lymphocytes) was observed from CIN III epithelium to cervical cancer tissue samples (Figure 2).
Cases (number) |
§NK cells (*) |
§Granz B+ (*) |
#Area (in mm2) |
Control (5) |
3 |
4 |
193,660.92 |
CINI (5) |
12 |
80 |
433,571.30 |
CINII (5) |
13 |
78 |
601,674.20 |
CINIII (5) |
28 |
135 |
689,028.10 |
Cancer (5) |
11 |
38 |
360,234.50 |
Cancer (5) |
11 |
38 |
360,234.50 |
Table 1 Median values of Natural Killer (NK)-like and granzymeB (GranzB) positive cells in the cervical (i) epithelium from patients without cervical alterations patients, with CINI, CINII and CINIII, and (ii) stroma from patients with cancer.
(#) Mean values of the total area size analyzed (expressed per mm2);
(§) The numbers of NK-like and GranzB positive cells were quantified from each digitalized high-power field (i) within the entire lesioned area from CIN epithelium and normal samples, and (ii) in the surrounding stromal area from cancer tissues. Median values of positive cells number were expressed as median, range, first and third quartilesper mm2 of tissue areas. The Kruskal-Wallis (non-parametric) 1-way ANOVA test was used to determine significant differences among three and more groups. (*) P values of 0.031 and 0.008 were respectively found for different numbers of NK and GranzB cells detected among normal and lesioned (CINI, II, II and cancer) groups, as determined by the non-parametric statistical test.
Figure 2 Natural Killer-like and GranzB positive T-cells quantification in cervical tissues. Box and whisker plot showing the (A) NK-like and (B) GranzB positive cells quantification in the epithelium (Normal, CINI, CINII and CINIII), and the stroma (cancer) of cervical samples [median, range, first and third quartiles]. (A) P (*) values of 0.0278 and 0.0160 were found to the median of NK-like cells number quantified in normal versus CINII tissues, and in normal versus CINIII samples, respectively. (B) P (*) values of 0.0021, 0.012, 0.012 and 0.012 were found to the median of GranzB+ T-cells number quantified in normal versus CINI, CINII, CINII and cancer tissues, respectively. P values were determined by the Kruskal-Wallis non-parametric statistical test analysis.
Box and whisker plot showing the (A) NK-like and (B) GranzB positive cells quantification in the epithelium (Normal, CINI, CINII and CINIII), and the stroma (cancer) of cervical samples [median, range, first and third quartiles]. (A) P (*) values of 0.0278 and 0.0160 were found to the median of NK-like cells number quantified in normal versus CINII tissues, and in normal versus CINIII samples, respectively. (B) P (*) values of 0.0021, 0.012, 0.012 and 0.012 were found to the median of GranzB+ T-cells number quantified in normal versus CINI, CINII, CINII and cancer tissues, respectively. P values were determined by the Kruskal-Wallis non-parametric statistical test analysis.
Cell-mediated immune responses are directed by CTLs and NK cells which represent important mechanisms for the elimination of neoplastic cells. However, evaluation of cytotoxic lymphocyte’s subpopulations in patients with cervical invasive and pre-invasive lesions has been reported only in few papers, which were mostly focused on the analysis of circulating lymphocytes.20-23 One study which performed by flow cytometry the peripheral blood lymphocytes (PBLs) analysis of 16 patients with SILs, and of 15 controls with normal cytology did not observe any differences in the number of TCD4+, TCD8+ and NK cells between the two groups. The authors concluded that disturbances in the immune response should probably be sought in the local defense mechanism of cervical epithelium and stroma.20
Despite the relevance of the cytotoxic functions of Natural killer cells and CTLs in the control of CIN progression and cancer, few reports were directed to investigate the density of NK cells in cervical tissues samples.13,14,18,24,25
Our study demonstrated an important increase in the number of NK-like cells in cervical tissues from patients with CIN and invasive cancer compared with tissues obtained from women without cellular abnormalities. One work based on the semi-quantitative analysis of NK cells detected by IHC in twenty-three cervical tissues observed the presence of NK cells in 2 out of 5 (40%) normal controls, in 5 out of 6 (84%) HPV-positive samples and in 9 out of 12 (75%) CIN tissues, with predominant location of NK cells in the sub-epithelial stroma.13 Another study evaluated by IHC the expression of two molecules involved with CTL activation, the TIA-1 and the granzyme B, which are only expressed in CTLs upon activation. Analysis of 24 CIN lesions with increasing atypia degree, and of 14 cervical carcinoma tissues revealed that only a minority of CTLs were activated in most CIN lesions, whereas a massive infiltration of activated CTLs (i.e. granzyme B-positive) was observed in some carcinomas.14
An important increase in the number of GranzB positive cells was detected in pre-neoplastic lesions, but especially in CINIII cervical tissues. These findings confirm those registered in previous studies using the specific marker for NK cells, the CD56, which showed increased number of CD56+ cells in HPV-related pre-neoplastic lesions.24,25 The observed increase in the density of Natural killer cells and of GranzB+/CD8+T-cells, in tissues from patients with high-grade lesions as CINII and III suggest the occurrence of a massive cytotoxic T-lymphocytes recruitment to cervical tissues so as to possibly control the events promoting cellular alterations and interrupting, in this way, CINIII progression towards to more severe lesions.9
Natural killer cells have been considered one of the most important cells involved in the control of HPV infection in cervical tissues. PCR detection of HPV-DNA in the cervical samples analyzed in this study by using a sensitive Nested-PCR protocol revealed the presence of HPV-DNA in 19 (95%) out of the 20 samples with SIL and cancer (data not shown). HPV16-DNA was observed in 20, 40 and 60% of patients with CINI, II and III, respectively, whereas 40% of CINIII patients were positive for HPV18-DNA. Twenty percent of patients with CINIII also exhibited HPV35- and HPV58-DNA. Additionally, DNA from HPV35, HPV45 and HPV52 was found in 60%, 40% and 20% of cervical tissues from patients with CINI, respectively (data not shown). Therefore, the observed high incidence of oncogenic HPV types in high-grade lesions as CINII and CINIII reinforce our results concerning the NK cells increase in high-grade SIL tissues, and suggest the involvement of cytotoxic NK and CD8+T-cells in the control of HPV infection and CIN progression.9,26,27
Tumor-infiltrating and, to a lesser extent, peripheral lymphocytes obtained from cancer patients and maintained in in-vitro cultures are known to have a poor immune response which can be normalized upon culture with recombinant pro-inflammatory interleukins (IL) as IL2. A multitude of different mechanisms could account for these observations, including decreased production of growth-promoting cytokines and/or the presence of immuno-suppressive cytokines.28,29 Consequently, growing interest on the investigation of functional aspects of T-lymphocytes and NK cells has been noticed in the last twenty years, through studies which were mainly focused on the functional analysis of peripheral lymphocytes.21,30
In one study based on the Fluorescent-activated Cell Sorting (FACs) analysis, the expression of molecules involved in the TCR signal-transduction and sequential activation of T-lymphocytes and NK cells (such as the TCR-cytoplasmic domains CD3 zeta (ζ), CD16 (ζ) and CD3 ε chains) was investigated in circulating lymphocytes.21 Decreased expression of CD3ζ chains has been related with reduced proliferative responses following antigenic challenge, and with decreased cytokine production in several tumor types and derived-cell lines such as renal cell carcinoma, melanoma, ovarian, colon and upper-gastrointestinal cancer.31,32 Accordingly, an important decrease in the CD3ζ chain expression was detected in PBLs isolated from 22 patients with cervical cancer and from 23 patients with SILs, as compared to PBLs obtained from 21 healthy donors. Reduced expression of CD3ζ chains was also observed in CD16+NK cells but only in PBLs obtained from patients with cervical cancer.21 The CD3ζ chain expression significantly correlated with the PBLs ability to produce Tumor Necrosis Factor (TNF) α in response to anti-CD3 stimulation.21 These data suggest that, alterations in the expression of zeta signal-transducing molecules commonly occur in patients with cervical cancer, and less frequently with those with SILs, and that the reduced zeta-chain expression could be associated with altered cellular functions such as TNFα production.21,23
The cytotoxic effect of NK cells against tumor and virus infected cells is thought to be a consequence from a fine balance between activating and inhibitory receptors. Accordingly, expression of triggering receptors as NKp30, NKp44, NKp46 and NKGD2 on the membrane surface of NK cells correlates with cytolytic activity against tumor cells.23 The expression of NKp30, NKp44, NKp46 and NKGD2 receptors in circulating lymphocytes was evaluated for the first time by FACs analysis on NK cells obtained from 59 patients with cervical cancer and SILs.23 The NK cell-activating receptors NKp30 and NKp46 were significantly down-regulated in cervical cancer and high-grade SILs patients. NKG2D was also down-regulated but only in PBLs from patients with cervical cancer. Importantly, the reduced number of NK expressing cell-activating receptors correlated with low-cytolytic activity, HPV16 infection and clinical stage of cervical disease.23 However, the functional aspects of the cytotoxic cells-GranzB+ analyzed in our study have not been performed.
The immune system controls in most situations the viral infection in the uterine cervix and, consequently, around 90% of HPV-infected women clear the virus within 2 years. Therefore, to understand the interactions between HPVs and immune cells is important to dissect the mechanisms responsible for the viral clearance observed in the majority of patients with SIL. However, it remains unclear which immune cells are implicated in this process, since the direct interaction between the HPV and NK cells has not been previously performed. An elegant in-vitro study evaluated the NK-cell response against HPV, based on the infection of NK cells isolated from healthy donors and maintained in culture by HPV Virus-like particles (VLPs). At the presence of HPV-VLPs, NK-cells displayed a higher cytotoxic activity (by increasing the exocytosis of their cytotoxic granules) and increased production of pro-inflammatory cytokines (TNF-α and INF-γ). By using flow-cytometry and electronic microscopy approaches, the NK-cells stimulation was linked to a rapid VLP entry into NK cells by macropinocitosis.
Data obtained from our and from previously performed studies suggest that NK cells can participate in the high rate of spontaneous regression of HPV-associated pre-neoplastic lesions. NK cells present in these pre-neoplastic lesions might be activated by viral particles and kill HPV-infected cells. Alternatively, NK cells could help to induce an adaptive immune response against HPV by secreting cytokines as it has been formerly emphasized.16 A better understanding of the role of NK cells in HPV-associated lesions could help to design more effective vaccines and treatment strategies for pre-neoplastic and neoplastic cervical diseases.
This work was supported by the Brazilian funding agencies (CAPES, CNPq and FAPEMIG, MG, Brazil). KAS was supported by an under-graduation scholarship from FAPEMIG. DSC was supported by a PhD scholarship from the CAPES, Brazil.
For this study, the authors received financial support from Brazilian funding agencies (CNPq and FAPEMIG, MG, Brazil).
The author declares no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.