eISSN: 2377-4304


Millions of women start using an IUD because doctors tell them that IUDs are safe, effective and long‒lasting. Unlike the pill, vaginal ring or the contraceptive patch, IUDs are fit and forget methods. In many women, this statement is true. Many women are happy with their IUD and don’t complain. However, about 50 to 60% have their IUD removed long before the usual 5‒year lifespan of the IUD. Early IUD/IUS removal is frequent due to side effects, mostly bleeding and pain, because the uterus, like any organ, cannot tolerate a foreign body that is cumbersome for long periods of time. Young women are specifically vulnerable to side effects. They are also the most vulnerable to unintended pregnancy and this is precisely why many organizations and institutions advocate IUDs in order not to become pregnant unintendedly. However, early IUD discontinuation undermines their potential to prevent unintended pregnancy and has numerous drawbacks as many women switch to other, less effective methods or to no method at all. Continuation over time is the primary determinant of effectiveness for IUDs.
As researchers, active in the field of intrauterine contraception since several decades, we have tried to maximize continuation of use by designing non‒hormonal and hormonal intrauterine devices that accommodate to every anatomically normal uterus. Frameless devices are small; they are flexible as they have no frame; they provide highly effective and well tolerated contraception simply because they fit. Embedment, a frequent complication of current framed IUDs, is not possible with the frameless IUD as the uterine contractions have no impact on the flexible body and can therefore not be forced in the uterine wall. However, frameless IUDs need to be attached to the uterus to prevent expulsion. Precisely how correct anchoring is accomplished, including the pitfalls, and how to check the correct position of the anchored IUD is the subject of this paper.
The intrauterine contraceptive devices (IUD) are considered important in reducing unintended pregnancy rates.1 IUDs are useful immediately following induced first‒trimester abortion, resulting in significantly fewer repeat abortions.2 In addition, copper IUDs are more effective than emergency contraceptive pills, providing long‒term contraception, if well tolerated.3 They are also the most cost‒effective contraceptive methods.4
However, their utility to reduce or eliminate unintended pregnancy depends on women or couples continuing to use the method. Compatibility between the IUD and the uterine cavity relationship, often overlooked by health care providers, is paramount to achieving this aim. As the design characteristics of IUDs differ greatly, patient individualization with respect to size and uterine fit has not been easily achieved. The following section reviews the concept of uterine compatibility as it relates to IUD design.
Many years ago, the importance of an optimal interrelationship between the IUD and the uterine cavity was stressed by IUD developers as fewer side effects and greater acceptability would thereby be promoted.5 They concluded that side effects such as pain during use of the IUD is related to a disproportion between the size of the uterine cavity and that of the IUD. Particularly a too wide IUD was found to be cumbersome (Table 1).
|
Method Used |
Hysterography |
Cavimeter |
3D Ultrasound |
2D Ultrasound |
|
FUD* |
23.1±3.1.5 |
23.5±0.9.6 |
27.1 (20.2‒34.1).7 |
24.4 (13.8‒35.0).8 |
Table 1 The width of the uterine fundus measured by different methods in nulliparous women
*Mean±SD; Mean (Range)
Ultrasound techniques, particularly sono‒hysterography, gel‒infusion sonography (GIS) and 3D ultrasound allow precise measurements (Figure 1).
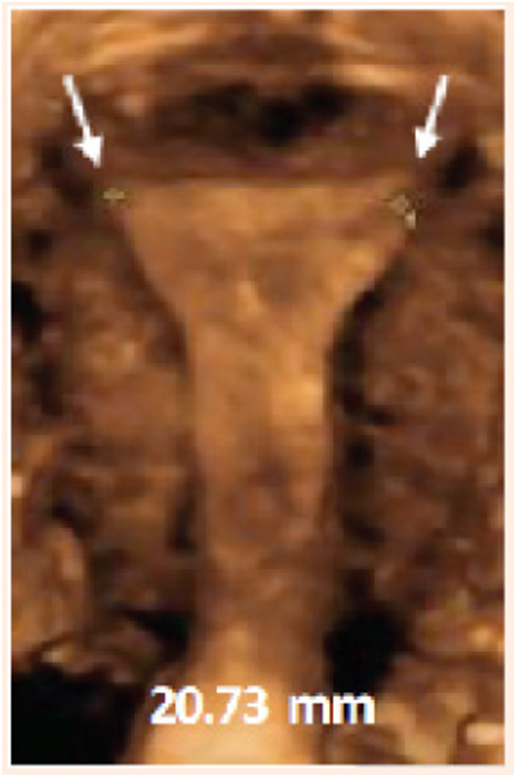
Figure 1 3D ultrasound illustration of the measurement of the transverse width of the uterine cavity (arrows show the transverse distance which is 20.73 mm in this case).
To assess the size and shape of the uterine cavity, 3D ultrasound is the easiest and most reliable method to also diagnose uterine anomalies or other gynecological conditions such as adenomas which may affect IUD/uterus compatibility. Unfortunately, screening for congenital or gynecological uterine anomalies is not practical to carry out routinely but may occasionally still have substantial clinical impact in the selection of an appropriate IUD. Overall, about 5.5% uterine anomalies are diagnosed in an unselected population. Arcuate uteri are the most frequent abnormalities affecting 3.9% of all women (Figure 2). Subseptate or septate uteri have a prevalence of 2.3%. Bicornuate uteri are uncommon (0.4%) and 0.1% of cases present a unicornuate uterus. Uterus didelphys is rare and occurs in only approximately 0.3% in an unselected population.9,10 ESHRE (The European Society of Human Reproduction and Embryology) and the ESGE (European Society of Gynecological Endoscopy) proposed a classification system to provide a comprehensive clinical orientation of congenital anomalies of the uterus. Anomalies are classified into the following main classes based on anatomical deviations derived from the same embryological origin (Figure 3) U0 or normal uterus; U1 or dysmorphic uterus; U2 or septate uterus; U3 or bicorporeal uterus; U4 or hemi‒uterus.11
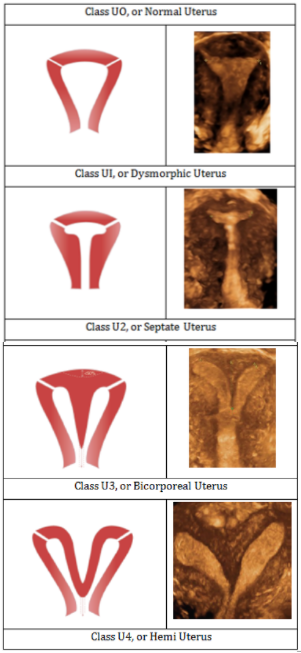

Figure 3 Classification of main uterine anomalies adapted from ESHRE/ESGE,12 schematic representation (left); 3D sonographic images (right).
During the different phases of the menstrual cycle uterine contractions can modulate the relationship between the IUD and the host endometrial cavity. Uterine contraction frequency is increased during the follicular phase, followed by a period of uterine quiescence during the luteal phase.12 Figure 4 shows the anatomical and functional changes of the uterine cavity during the cycle. These contractions can compress, distort, displace, and expel the IUD, particularly if the IUD is too big and is not capable of adaptive changes.9 The impact of the uterine forces can be quite severe as illustrated in Figure 4.
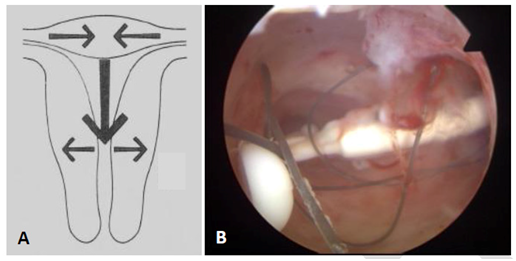
Figure 4 A) Anatomical and functional changes of the uterine cavity on cycle day 1 contracted fundus with reduced transverse diameter; relaxed isthmus with increased transverse diameter; definite fundus‒to‒cervix muscle propagation waves.9 B) The effect of uterine forces on a Mirena IUD causing transverse positioning of the IUS with embedment.
In premenopausal women, an enlargement in uterine size is observed between nulliparous and parous women. The increase in volume is attributed predominantly due to an increase in thickness of the uterine wall since the uterine cavity width does not change much.7,13 A significant increase of the uterine volume occurring towards the end of the menstrual cycle is also observed.14 (Table 2).
|
Gravidity/Parity (n [%]) |
0 (n = 91) |
1 (n = 38) |
>1 (n = 81) |
|
Mean volume* (cm3) (SD) |
55.3 (25.7) |
66.4 (29.2) |
103.1 (33.1) |
Table 2 Uterine volume according to gravidity/parity (3D measurements).7
The length of the IUD is clinically of lesser clinical importance, however it may contribute to uterine compatibility.15 Approximately one third of uterine cavities of nulliparous women are shorter than the length of the stem of the current IUDs.8 When the length of the stem is equal or longer to that of the endometrial cavity, irritation of the isthmus region will trigger myometrial contractions that promote pain, translocation, expulsion or embedment.
Implications for IUD Users
The mean transverse dimension of uterine cavities in parous and nulliparous women are far less than the length of the transverse arm of most used conventional IUDs (e.g., Paragard IUD and Mirena levonorgestrel intrauterine system). The transverse arm length of these devices is 32 mm, which is too long for many uterine cavities resulting often in distortion, displacement, and expulsion of the IUD. The length of the devices are 36 and 32 mm, respectively.
Spatial incompatibility can be circumvented by adaptation of T‒shaped IUDs (Figure 5). If the IUD fits, IUD acceptance will be enhanced, thus maximizing continuation of use. These optimal geometric relationships promote IUD retention and stability while minimizing endometrial/myometrial trauma.
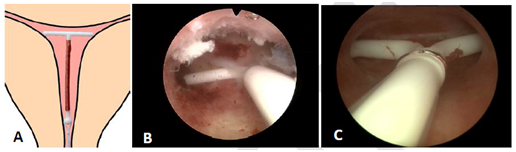
Figure 5 A) Adapted T‒shape intrauterine device with transverse arm of 18 mm; B) Hysteroscopic view of an LNG‒IUS in situ with 6 mm shortened transverse arm. Note the optimal spatial compatibility with narrow uterine cavity; C) The smaller Jaydess/Skyla LNG‒IUS with both arms embedded in the uterine wall.
Precision Intrauterine Contraception to Promote High Continuation of Use
Comfort during IUD use and a high continuation of use is obtained by using an IUD that is not significantly wider than the width of the uterine cavity. The new Jaydess (Skyla® in the USA), having a transverse arm length of 28 mm is conceived for that reason. Initial clinical trials are encouraging,16 but this 28 mm transverse arm may still be too long for many women as the IUD cavity width is less than 24 mm in many women. Figure 6 illustrates the width of the uterine cavity in nulliparous women.
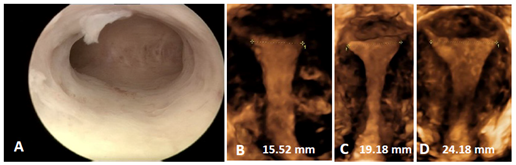
Figure 6 A) Hysteroscopic picture of a narrow uterine cavity. The cavity is slightly less than 20 mm; B, C and D) 3D ultrasound pictures of uterine cavities focused on the cavity width which corresponds with that of the majority of nulliparous women.
The frameless copper IUD (GyneFix)
Uterine cavities differ considerably in size and shape (Figure 7).6,17
Figure 7 Schematic representation of the differences in uterine cavities A) Differences in width; B) Differences in length; C) Functional changes in size and examples of incompatibility. The width of the cavity is the most important parameter related to IUD performance and tolerance.6
T‒shape designed IUDs rely on a crossarm width of 32mm (some 28mm) which is much greater than the mean uterine diameter of 24mm.6,8 Their size is less than optimal for many women, and will lead to patient discomfort, pain, embedment and possibly uterine perforation, particularly if there is a large disparity between the IUD and the narrow uterine cavity.7,18 The “frameless” IUD, has a diameter which is small and can therefore be used in all uterine cavities. The frameless IUD has been successfully inserted and well tolerated in women with a very narrow uterine cavity (Figure 8).19
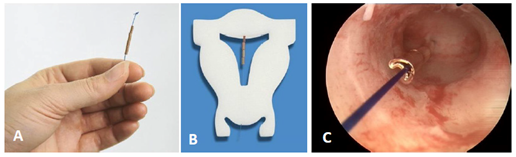
Figure 8 A) Frameless copper IUD with anchoring knot on the upper end. The anchor is inserted in the fundus of the uterus with a specially designed inserter. The IUD consists of hollow copper tubes which release copper ions from the outside and the inside. B) Frameless IUD inserted in a foam uterus. C) Hysteroscopic view of the frameless IUD attached to the fundus of this (narrow) uterine cavity.
An IUD that fits could significantly contribute to all current efforts to reduce the number of unintended pregnancies, particularly in young nulliparous and adolescent women, as this will enhance tolerance and continuation of use. However, too large IUDs will compress, distort, displace, and expel the IUD. This will result in early discontinuation of use (Figure 9). Moreover, displaced IUDs in the lower uterine segment or the cervix result in higher pregnancy rates and should therefore be removed or replaced.20,21
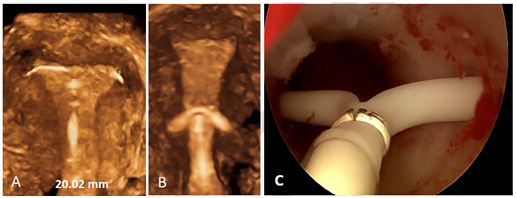
Figure 9 A) 3D ultrasound pictures of T‒shaped Jaydess IUD, apparently in normal position but with crossarms embedded in the uterine wall causing pain during sexual intercourse. The width of the uterine cavity is 20.02 mm; B) This uterus is only about 16 mm wide whilst the length if the crossarm is 32 mm; C) LNG‒IUS displaced and embedded in cervix.
The characteristics of the frameless IUD, being totally flexible, eliminate the ability of the uterus to exert expulsive forces on the frameless IUD. The attachment to the fundus of the uterus minimizes the risk of expulsion.22,23 Long term comfort, especially for those women (e.g., nulliparous and adolescent women) with a small or distorted uterine cavity, and for women who have experienced problems with framed IUDs can be achieved with a frameless IUD (Figure 9).19,24
Copper IUDs increase menstrual blood loss causing anemia in many of them (Figure 10).25 Large intrauterine devices appear to result in a greater amount of blood loss during menses.26 By reducing the surface area of the copper IUD, menstrual blood loss can be minimized. The frameless IUD design allows copper release from both the interior and exterior surfaces of the hollow copper tubes, thereby reducing the size of the IUD. Menstrual blood loss studies did not show a significant increase in menstrual bleeding with the small frameless IUD.27
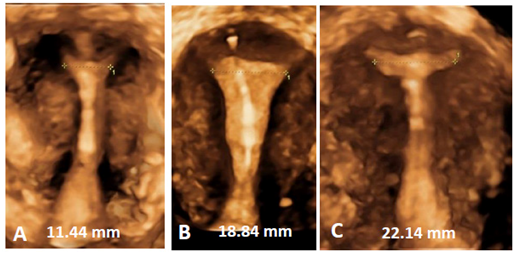
Figure 10 A, B and C) 3D ultrasound pictures of the frameless copper IUD in three uteri with width between 11.4 mm and 22.2 mm. Frameless IUDs accommodate cavities of different sizes and shapes maximizing performance and resulting in long duration of use.
How to insert the frameless IUD
Supervised training in a model (HUT®), the home uterine trainer, is important to become familiar with the anchoring technique of insertion of the frameless IUD.
*Note Much of the increase in uterine volume occurs after pregnancy and is due to the uterine muscle which becomes thicker; the cavity, however, doesn’t change much.
SD Standard Deviation
Before insertion Studies have shown that cervical traction in a caudal direction reduces the median uterocervical angle, from 75° to 10° and moderate cervical traction straightens the uterus, and the routine use of a tenaculum theoretically should make insertion of an IUD safer.28 A prerequisite, however, is that traction should be applied until the insertion of the IUD has been completed. Figure 11A & 11B show the impact of traction on the uterus in relation to the insertion site of the uterus. Slight traction on the cervix throughout the procedure ascertains proper positioning of the anchor in the midline of the uterus. The fundus is thicker in the midline than close to the fallopian tubes (Figure 13B).
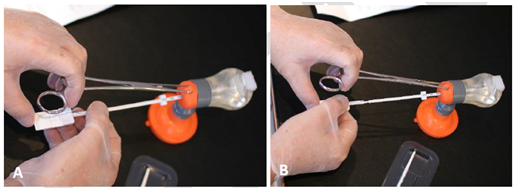
Figure 11 Make it simple A) If you position your hand on the short tenaculum as shown, and. B) if you then move the handle gently and controlled forward, while you continue to exert traction on the cervix to aligning the uterus, you will accomplish a perfect insertion. This will help minimizing failed insertion as you go straight to the middle of the fundus which is the thickest part. Feeling the anchor penetrating the fundal tissue will provide additional confidence.
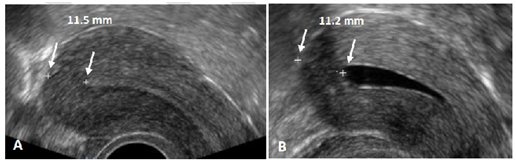
Figure 12 A) Example of measurement of the thickness of the uterine fundus. In this case the fundus is 11.5 mm thick. From time to time, it is more difficult to measure the fundal thickness as there may be less contrast between the endometrium and myometrium. B) Ultrasound measurement in the second half of the cycle may provide better results, or a 2D ultrasound measurement may be performed after injection of gel in the uterine cavity to allow precise measurement of the thickness of the fundus (Gel infusion sonography or GIS).
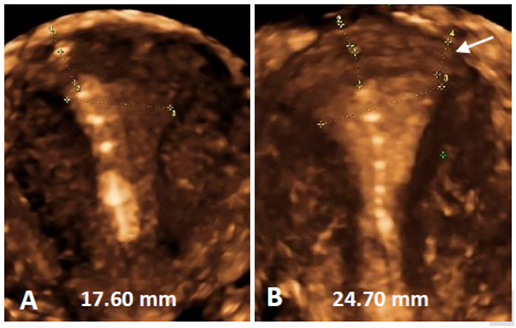
Figure 13 A) If no traction throughout the procedure is exerted there is a risk of oblique insertion or insertion in the anterior, posterior or lateral wall of the uterus. Note the anchor below the serosa; B) Traction throughout the procedure will avoid oblique insertion which could be a reason for perforation as the fundus can be thinner at the level of utero‒tubal junction. The thickness in the midline is 11 mm whilst it is only 6.8 mm close to the tubal ostium (see arrow).
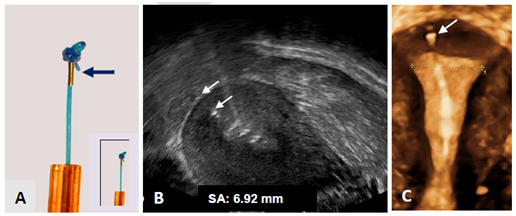
Figure 14 A) Anchor with visualization element (magnification x 2.5) Tiny metal piece (2 mm long and 0.5 mm in diameter; insert real size); B) 2D ultrasound of visualized anchor located 6.92 mm from the serosa of the uterus (arrows); C) 3D ultrasound showing the frameless IUD respecting the anatomical configuration of the small uterine cavity. The anchoring knot is placed in the fundal tissue (arrow) assuring proper retention of the IUD.
Providers should be cautious in women using the three‒monthly injectable as they can have a very thin fundus. Insertion in these patients should therefore be postponed until the fundus is sufficiently thick. In addition, postpartum lactating women may have a soft uterus which could be a risk factor for perforation of the uterus. A period of at least 2‒3 month should be awaited until the uterus is completely returned to normal size.
Clinical experience shows that access to the uterus, and straightening of the utero‒cervical axis, is facilitated by using the lithotomy position for all IUD/IUS insertions. In overweight and obese women, if the woman pulls at her knees visibility is improved tremendously.
The risk of perforation is minimized by assessing the thickness of the fundal wall of the uterus prior to insertion of the frameless IUD. The thickness of the fundus should be at least 10 mm in the midline. Assessing the thickness by ultrasound may help in providing a safe insertion. The use of a forceps with 18 or 19 cm in length, Allis or Pozzi forceps, is recommended as this facilitates insertion. Figure 12 gives an example of the measurement of the thickness of the fundal wall.
How to avoid failed insertion‒expulsion‒perforation? Insertion is as simple and straightforward as inserting a uterine sound. Failed insertion/expulsion is usually the consequence of inadequate insertion of the anchor in the myometrium of the uterine fundus. Recommendations and advice is given in Table 3. Midline insertion is easily accomplished if traction on the cervix is exerted throughout the insertion procedure.
|
1. |
Measure the thickness of the uterine fundus The thickness of the fundus should be at least 10 mm. If slightly less then 10 mm, insert the anchor slowly and gently, feeling the anchoring knot penetrating the muscular wall. |
|
2. |
Use of a forceps with 18 to 19 cm in length, Allis or Pozzi forceps, is recommended as this facilitates insertion and aligning the utero‒cervical axis. |
|
3. |
Use a short speculum to have close access to the cervix of the uterus. |
|
4. |
Perform the “cotton swab test” This test with a cotton swab soaked in antiseptic solution can be done to test the tightness of the internal cervical os and to obtain information on pain sensation. If the test provokes severe pain, local anesthesia can be provided prior to sounding the uterus. Additional instrumentation (e.g., os finder, cervical dilator), except for a sound to measure the length and flexion of the uterus, is rarely necessary if the test shows that the cavity can be entered easily. |
|
5. |
Attention to comfort during insertion is very important. If necessary, perform cervical priming by, for example, Cytotec® 1 to 2 tablets tablets of 200mg orally, 3 hours before the insertion. In addition, you can also use intra‒cervical anesthesia, preferably with a dental syringe. |
|
6. |
Following delivery, especially in breast‒feeding mothers, the insertion should be postponed until three months after birth. |
|
7. |
Women using the 3‒monthly injectable should not use GyneFix as long as the uterus is atrophic. |
|
8. |
After the insertion, the woman should not have intercourse and not use tampon/mooncup within 7 days. |
Table 3 Recommendations before insertion and some advice
After insertion The visualized anchor is a key element in the optimization of the frameless technology with the aim to allow the provider to check its placement at insertion and at follow‒up which enhances provider confidence and assurance. Figures 13 and 14 illustrate the measurement of the SA‒distance.
The frameless IUD is a unique IUD which, if correctly inserted, will be well tolerated by the majority of women, also nulliparous and adolescent women. Many adolescent and nulliparous women prefer the IUD over non‒LARC methods when they are properly informed about the advantages over short‒acting methods. They are interested in safe, effective, well tolerated, and long‒acting contraception. The late Dr Harrith M Hasson who was honored for his research on the uterine geometry related to IUD performance concluded that”…with few exceptions, the performance record of a IUD is basically determined by its geometric relationship to the host uterine cavity”.
Measure→Feel→Measure Measuring the thickness of the fundus provides assurance; feeling the anchor penetrating the muscle wall provides confidence and measuring the SA‒distance confirms proper positioning in the fundus of the uterus.
In order to increase women’s contraceptive choices and reduce the rate of unintended pregnancy, expanding IUD access should be a public health priority. Decreasing physician and patient anxiety about the risks of IUDs is paramount to achieving that goal. Below in Table 4 are the two most important misconceptions.
|
IUDs cause pelvic inflammatory disease |
The issue of increased risk or greater severity of sexually transmitted infection (STI) among IUD users has been a prominent concern. However, the rate of pelvic inflammatory disease (PID) is low, with cases concentrated in the first 20 days after insertion. The reason for the increased risk during the first weeks after insertion is that bacteria in the vagina and cervix can be transported through the cervical canal into the uterine cavity. It is important to tell the IUD user that for the majority of the users, fertility is restored immediately after removal of the device; irrespective of whether the IUD was used for a few months or for many years.29,30 |
|
IUDs cannot be used by nulliparous women |
Another myth is that women over 25 years or older are the best candidates for IUD use, and that women over 35 are the ideal candidates. This belief, based on the fear of pelvic infection (PID) and the potential for resulting infertility, is no longer justified. There is no biological reason to conclude that a young woman is at higher risk than an older woman provided they have similar sexual behaviors.31 |
Table 4 The two most important misconceptions

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.