Open Access Journal of
eISSN: 2576-4578


Research Article Volume 2 Issue 4
1Department of Veterinary Physiology-Biochemistry, Central Agricultural University, Selesih 796014, Aizawl, India
210 Maharana Pratap Colony, Sector 13, Hiran Magri, Udaipur-313 002, India
3Department of Pharmacy, Regional Institute of Paramedical and Nursing Sciences, Zemabawk 796017, Aizawl, India
4Department of Radiotherapy, Regional Institute of Medical Sciences, Imphal 795001, India
Correspondence: Ganesh Chandra Jagetia, 10 Maharana Pratap Colony, Sector 13, Hiran Magri, Udaipur-313 002, India
Received: May 21, 2018 | Published: September 5, 2018
Citation: Shantabi L. Jagetia GC, Devi NB, et al. Croton caudatus Geiseler toxicity evaluation in Swiss albino mice transplanted or not with Dalton’s lymphoma ascites tumor before exposure to 6 Gy γ-radiation. Open Access J Trans Med Res. 2018;2(4):109-116. DOI: 10.15406/oajtmr.2018.02.00050
The acute toxic effects of different doses of various extracts of Croton caudatus leaf (CCE) was studied in mice. The intraperitoneal administration of different CCEs showed a dose dependent increase in the acute toxicity in mice. The toxic effects of aqueous extract were highest when compared to the chloroform and ethanol extracts of C. caudatus. The LD50 was 0.35 g/kg b. wt. for aqueous CCE, whereas it was 0.5 g/kg b. wt. for chloroform and 0.65 g/kg b. wt. for ethanol extracts, respectively. The toxic effects of ethanol extract of Croton caudatus were further confirmed by estimating various biochemical parameters in non-tumor bearing as well as mice transplanted with Dalton’s lymphoma tumor. The administration of different doses of CCE with or without irradiation showed a dose dependent rise in the toxicity in both normal and Dalton’s lymphoma bearing mice as indicated by higher activities of alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase enzymes in mouse liver along with the elevation of albumin, total proteins, and total bilirubin contents. Similarly, kidneys of ethanol CCE treated mice showed increased level of urea, uric acid and a reduction in creatinine indicating infliction of biochemical lesions in these organs.
Keywords: Croton caudatus, LD50, acute toxicity, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and Dalton’s lymphoma.
Radiotherapy is one of the important modalities to treat malignant diseases. Radiotherapy alone is not enough to control tumor therefore, it is usually combined with other modalities to treat difficult neoplasia in the clinical condition. Radiotherapy is usually combined with chemotherapeutic agents to get better response and cure rates in patients.1 Despite the fact that radiotherapy is used to treat approximately 50% tumors, the induction of radiotoxicity in the normal cells remains the major limiting factor. Kim et al.2 The curative effects of irradiation are temporary and are highly localized to the irradiated area. It is well known that irradiation causes long term adverse side effects on body’s healthy normal tissues. The radiotherapy usually leads to tiredness, feeling of run down, sore throat, difficulty in swallowing, cough, hair loss, chest pain, increased body, temperature, shivering, sick feeling and sore skin.3 Radiotherapy uses high-energy X- or γ- rays to kill cancer cells and shrink tumors. Ionizing radiations belong to electromagnetic radiation category, and their wavelength is very short that make them highly effective. The major cell killing effect of radiotherapy is brought by causing various types of DNA damages and in particular DNA double strand breaks in cells.4 This indicates that tumor shall be made more vulnerable to radiation so as to spare the normal cells. Combination chemotherapy or chemoradiotherapy has been tried to treat difficult neopalsia and several combinations of chemotherapeutic drugs are used as cancer treatment regimens.5The combination chemoradiotherapy is also utilized in an attempt to increase the effectiveness and reduce the radiotoxicity in the patients by lowering the dose of the agent/s.6 Ttraditionally Croton caudatus Geiseler (family Euphorbiaceae) or kam-sabut has been utilized to treat several disorders including sprains, fever and liver diseases in the northeastern part of India. Its roots have been found to be purgative and it has low toxicity.7The leaves are applied on festering wounds of injured cattles and to ward off against the maggots. Our earlier studies have shown that kam-sabut acts as an antioxidant at low doses Shantabi et al.8 Therefore, the aim of this study was to examine various biochemical lesions induced by kam-sabut in the liver and kidney of Dalton’s lymphoma transplanted mice following exposure to 6 Gy γ- radiation.
Collection and Extraction of Plant Material
The mature and non-infected leaves were plucked from Croton caudatus Geiseler (Family: Euphorbiaceae) plants growing at Saikot, Churachandpur District of Manipur in the dry season. The kam–sabut was identified by Professor Kumar Singh, a well-known taxonomist of Manipur University, Imphal, India, and was further authenticated by the Botanical Survey of India, Shillong. The leaves were cleaned shade dried at room temperature and powdered in a grinder at room temperature. Usually 100g of leaf powder was sequentially extracted in petroleum ether, chloroform, ethanol and water using a Soxhlet apparatus until the solvents became clear. The extracts were concentrated by drying under reduced pressure and stored at -80 until further use. Henceforth the chloroform, ethanol and water extract/s of Croton caudatus will be called as CCC, CCE and CCA, respectively.
Animal care and handling
The animal care and handling were performed in keeping with the guidelines issued by the World Health Organization, Geneva, Switzerland, INSA (Indian National Science Academy, New Delhi, India) and the “Guide for the care and use of Laboratory Animals” (National Academy of Sciences, USA, 2010). Ten to twelve weeks old male and female Swiss albino mice weighing 30 to 36 g were selected from an inbred colony maintained under the controlled conditions of temperature (23±2°C), humidity (50±5%) and light (12h each of light and dark, respectively). The animals had free access to the sterile food and water. Four animals were housed in a polypropylene cage containing sterile paddy husk (procured locally) as bedding throughout the experiment. The study was approved by the Institutional Animal Ethical Committee of Regional Institute of Medical Sciences, Imphal, India.
Preparation of drug and mode of administration
The chloroform extract of Croton caudatus (CC) was freshly dissolved in 100 ml of chloroform and diluted with sterile physiological saline (SPS) containing 1.0 % carboxymethylcellulose (CMC). The ethanol extract of Croton caudatus was dissolved in 5% ethanol and diluted with sterile physiological saline (SPS) containing 1.0 % carboxymethylcellulose (CMC). Aqueous extract was dissolved in sterile physiological saline (SPS). Each animal from each group was injected with 0.01ml/gb wt. of either chloroform, ethanol or aqueous extract intraperitoneally.
Acute Toxicity
The acute toxicity of CCE (Croton caudatus extract) was determined.9 according to OECD guideline No. 420–425 (2001). Both male and female albino mice were randomly selected from the colony and assigned individual identification number. The animals were fasted for 18 h and were divided into three groups. Thereafter the animals were administered intraperitoneally with 0.25, 0.5, 1.0, 2.0, 3.5, 4.0, 5.0 g/Kg body weight of different CCEs, whereas the matching control group received SPS containing 1.0 % CMC. The animals were provided with food immediately after administration of different extracts. Animals were observed continuously for first two hours and every 6 hours until 24 hours, and daily thereafter, for a total period of 14 days for the development of toxic symptoms. The data were collected for the toxic manifestations including alteration in the behavior, tremors, convulsions, salivation, diarrhea, lethargy, sleep, and coma, time of onset of the symptoms and length of recovery period. The observations were recorded and individual records for each animal was maintained (Table 1) (Table 2) & (Table 3). Eight animals were used for each dose of each extract for this experiment and a total of 200 animals were used to complete the experiment.
Tumor model
Experimental tumor models have a played an important role in anticancer drug discovery. The Dalton’s ascites lymphoma (DAL) tumor transplanted into Swiss albino mice is a convenient model system that allows evaluation of antitumor activity of any pharmacological agent within a short time.10DAL procured from NEHU, Shillong, India, was maintained and propagated by serial intraperitoneal transplantation in an aseptic environment. Usually 106 viable DAL cells were injected intraperitoneally into each mouse in an aseptic condition and the day of tumor inoculation was considered as day 0. All the experiments in tumor bearing mice were carried out according to the standard protocol recommended by National Service Center of Cancer Chemotherapy, USA.11The tumorized animals were divided into the following groups after one day of tumor inoculation according to the treatment they received:
CCE + sham-irradiation This group of animals was administered with 0, 50, 75, 100, 125, 150, 175 or 200 mg/kg b. wt. of CCE once daily for consecutive nine days before sham-irradiation.
SPS + irradiation The animals of this group received 0.3 to 0.36 ml SPS 1 h before γ-radiation one day after tumor inoculation and then once daily for next eight consecutive days after irradiation.
CCE + irradiation The animals of this group were injected with 50, 75, 100, 125, 150, 175 or 200, mg/kg b. wt. of CCE 1h before γ-radiation one day after tumor transplantation and then once daily for subsequent eight consecutive days after irradiation.
Irradiation
The non-anaesthetized, tumorized, and prostate mice were restrained and immobilized (achieved by inserting cotton plugs in the restrainer) in a specially designed well-ventilated perspex box and the lower half of the animal body, below rib cage (hemi-body) was exposed to 0 (sham-irradiation) or 6 Gy γ- radiation as the case may be. Usually a batch of eight animals was irradiated at a dose rate of 2 Gy/min at source to animal distance (midpoint) of 78.9 cm using a Telecobalt therapy source (Theratron, Atomic Energy Agency, Ontario, Canada).
Biochemical analyses
For the biochemical assay the animals from each group were killed after nine days of irradiation. The animals were perfused with isotonic cold saline transcardially and their livers and kidneys were removed. Both the kidneys and livers were blot dried, weighed and 10% homogenate was prepared in PBS using a homogenizer (Remi, India). The aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP), albumin, total proteins and total bilirubin were estimated in the liver, whereas urea, uric acid and creatinine were measured in the kidney homogenate with the help of commercially available Response Kits using a Response 910 auto analyzer (Diagnostic Systems GmbH¸ Holzheim, Germany).
Statistical analysis
The statistical significance was determined using student’s’ test for biochemical studies. One-way ANOVA was used for multiple comparisons with the application of appropriate post-hoc test. The results were confirmed by repeating the experiments. Test of homogeneity was applied to determine any statistical differences between the repeat experiments. Since no significant differences were observed the data of all experiments were combined and expressed as mean ± standard error of the mean (SEM). A P of <0.05 was considered as statistically significant.
The results of acute CCE toxicity and biochemical changes are depicted in (Tables 1– 6) and (Figure 1–3) as mean ±SEM.

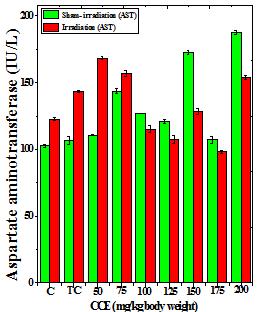
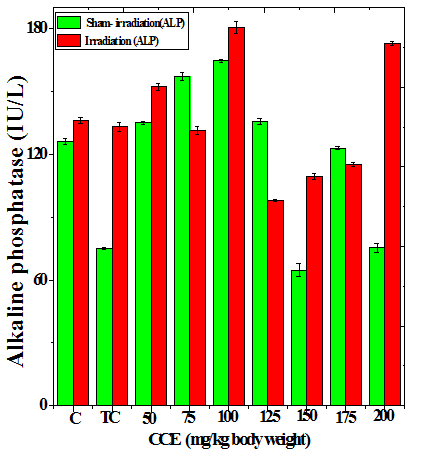
Figure 1 Alteration in the various enzymes activities in the liver of Dalton’s lymphoma transplanted mice treated or not with different doses of Croton caudatus ethanol extract (CCE) before exposure to 6 Gy γ-radiation. A: alanine aminotransferase (ALT) B: aspartate aminotransferase (AST) c: alkaline phosphate (ALP). C=Normal mice without DAL control; TC= DAL Tumor bearing mice control.


Figure 2 Alteration in the albumin (a) and bilirubin (b) profiles in the liver of Dalton’s lymphoma transplanted mice treated or not with various doses of Croton caudatus ethanol extract (CCE) before exposure to 6Gy γ-radiation.
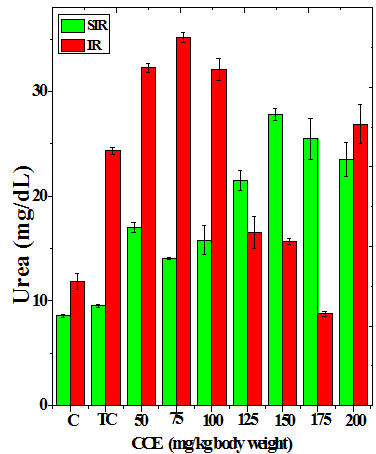
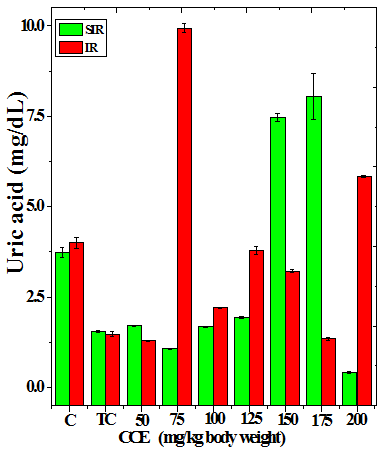

Figure 3 Alteration in the urea (a), uric acid (b) and creatinine (c) profiles in the kidney of Dalton’s lymphoma transplanted mice treated or not with various doses of Croton caudatus ethanol extract (CCE) before exposure to 6 Gy γ-radiation.
Acute toxicity
The animals treated intraperitoneally with CCE did not show any toxic symptoms or mortality up to a dose of 0.5 mg/kg body weight for ethanol and chloroform extracts except aqueous extract where the animal succumbed to death at a dose of 0.25 mg/kg body weight indicating maximum tolerated safe dose. A further increase in CCE dose caused increased mortality and no animals could survive beyond 50 mg/kg b. wt. of CCEs. (Tables 1– 3)
Dose(gm/kg) |
Mortality (%) on different days |
|
|
|
|
|
|
|
Remarks |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
Total |
||
0 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
0 |
Active and all survived. |
0.3 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
0 |
Active and all survived. |
0.5 |
12.5 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
25 |
12.5 |
- |
- |
50 |
Active and 4 died on 12 days. |
1 |
- |
13 |
13 |
- |
13 |
- |
13 |
13 |
13 |
13 |
- |
- |
- |
- |
87.5 |
Lethargic 50% died before 7 days. |
2 |
- |
38 |
- |
13 |
- |
13 |
13 |
25 |
- |
- |
- |
- |
- |
- |
100 |
Dull and died before 14 days. |
3.5 |
37.5 |
- |
- |
13 |
- |
- |
25 |
- |
13 |
13 |
- |
- |
- |
- |
100 |
Lethargic and died before 14 days. |
4 |
100 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
100 |
Aggressiveness, dullness and died within 4h. |
5 |
100 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
100 |
Aggressiveness, dullness and died within 2h. |
Table 1 Effect of chloroform leaf extract of Croton caudatus on the acute toxicity in mice
Dose(gm/kg) |
Mortality (%) on different days |
|
|
|
|
|
|
|
Remarks |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
Total |
||
0 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
0 |
Active and all survived. |
0.3 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
0 |
Active and all survived. |
0.5 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
13 |
12.5 |
- |
- |
25 |
Active and 25% died before 14 days. |
1 |
- |
13 |
13 |
- |
13 |
25 |
- |
13 |
- |
13 |
- |
- |
- |
- |
87.5 |
Lethargic and 50% died before 7 days. |
2 |
- |
25 |
- |
- |
- |
13 |
25 |
- |
38 |
- |
- |
- |
- |
- |
100 |
Dull and died before 14 days. |
3.5 |
- |
13 |
13 |
25 |
- |
25 |
13 |
13 |
- |
- |
- |
- |
- |
- |
100 |
Lethargic and died before 14 days. |
4 |
100 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
100 |
Aggressiveness, dullness and died within first day. |
5 |
100 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
100 |
Aggressiveness, dullness and died within 3h. |
Table 2 Effect of ethanol leaf extract of Croton caudatus on the acute toxicity in mice
Dose(gm/kg) |
Mortality (%) on different days |
|
|
|
|
|
|
|
Remarks |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
Total |
||
0 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
0 |
Active and all survived. |
0.2 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
0 |
Active and all survived. |
0.3 |
- |
- |
13 |
- |
- |
- |
- |
- |
- |
13 |
- |
12.5 |
- |
- |
37.5 |
Active and 37.5% died before 14 days. |
0.5 |
- |
13 |
- |
25 |
- |
13 |
- |
- |
- |
- |
- |
12.5 |
- |
- |
62.5 |
Active and 62.5% died before 14 days. |
1 |
- |
13 |
- |
13 |
13 |
13 |
- |
13 |
13 |
- |
25 |
- |
- |
- |
100 |
Lethargic and 50% died within7 days. |
2 |
- |
- |
13 |
13 |
- |
25 |
25 |
- |
25 |
- |
- |
- |
- |
- |
100 |
Dull and all died before 14 days. |
3.5 |
12.5 |
25 |
- |
13 |
13 |
38 |
- |
- |
- |
- |
- |
- |
- |
- |
100 |
Lethargic and all died before 14 days. |
4 |
100 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
100 |
Aggressiveness, dullness and all died within 1h. |
5 |
100 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
100 |
Writhing, aggressiveness, dullness and all died within 10 min. |
Table 3 Effect of aqueous extract of Croton caudatus on the acute toxicity in mice
Biochemical analysis
The treatment of tumorized mice with various doses of CCE caused alteration in the activities of ALT and AST which were higher than that of normal mice (Table 4), whereas the activities of ALP was higher only for 50, 75, 100 and 125 mg/kg CCE (Figure 1) in the mice liver (Table 4) (Figure 1). The combination of CCE and irradiation further increased their activities in tumorized mice significantly (Table 4) (Figure 1). However, there was no clear pattern of increase in these enzymes. Similarly, treatment of tumorized mice with CCE resulted in an increase in the albumin; total protein contents whereas bilirubin contents did not reveal any significant alteration (Table 5) (Figure 2). Irradiation of CCE treated mice reduced albumin and total protein contents whereas, bilirubin contents remained almost unchanged (Table 5) (Figure 2). The kidney function test showed a rise in the urea, and uric acid except creatinine, which was lower in tumorized mice (Figure 3). Administration of different doses of CCE into tumorized mice significant rise in the urea and creatinine contents, whereas uric acid higher for 100 and 125 mg/kg CCE (Table 6). Irradiation of tumorized mice increased the urea and creatinine and marginally and alleviated uric acid contents. Treatment of tumorized mice with different doses of ethanol CCE led to a further rise in the urea (except 125, 50 and 175 mg/kg) and uric acid (except 50 and 175 mg/kg) contents, whereas and creatinine levels were lower for all CCE doses (Table 6) (Figure 3).
CCE Dose (mg/kg) |
ALT (U/L) |
AST (U/L) |
ALP (U/L) |
||||
|---|---|---|---|---|---|---|---|
|
Sham-Irradiation |
Irradiation |
Sham-Irradiation |
Irradiation |
Sham-Irradiation |
Irradiation |
|
Normal |
27.83±0.49 |
47.83±0.49d |
102.7±1.30 |
122.7±1.30d |
126.1±1.56 |
136.1±1.56b |
|
Tumor Control |
72.26±0.14 |
95.16±0.66 d |
106.7±3.15 |
143.4±0.86d |
75.06±0.60 |
133.2±2.28d |
|
50 |
78.33±0.88 |
98.33±3.53b |
110.5±0.34 |
158.3±1.45d |
135.0±0.52 |
152.3±1.77c |
|
75 |
76.33±1.04 |
101.6±1.22d |
123.5±2.09 |
156.7±2.20d |
147.4±1.87 |
131.3±1.83b |
|
100 |
65.5±0.45 |
90.46±2.49c |
127.0±0.14 |
125±2.53 |
164.8±0.76 |
140.6±2.90d |
|
125 |
59.13±1.67 |
64.03±0.61 |
120.8±1.72 |
107.4±2.82a |
135.7±1.26 |
104.9±0.54d |
|
150 |
62.5±0.81 |
68±0.60b |
128.7±1.46 |
118.6±2.34a |
64.7±3.26 |
109.3±1.45d |
|
175 |
48.73±2.18 |
71.3±0.51c |
107.2±2.63 |
98.3±1.11a |
123.0±0.65 |
115.2±0.93b |
|
200 |
98.8±2.58 |
125.2±0.93d |
137.4±1.44 |
153.7±1.26c |
75.4±2.22 |
173±1.15d |
|
Table 4 Alteration in the biochemical profile in the liver of tumor bearing mice treated with different doses of Croton caudatus ethanol extract alone or in combination with 6 Gy of γ-irradiation
CCE Dose (mg/kg) |
Albumin ( g/dL) |
Total Proteins (g/dL) |
Total Billirubin |
|||||
|---|---|---|---|---|---|---|---|---|
Sham-Irradiation |
Irradiation |
Sham-Irradiation |
Irradiation |
Sham-Irradiation |
Irradiation |
|||
Normal |
0.13±0.01 |
0.14±0.01 |
0.57±0.02 |
0.57±0.02 |
0.07±0.01 |
0.07±0.01 |
||
Tumor Control |
0.48±0.02 |
0.11±0.00d |
0.98±0.01 |
0.46±0.02d |
0.06±0.00 |
0.05±0.00 |
||
50 |
0.27±0.01 |
0.18±0.02a |
0.75±0.01 |
0.31±0.01d |
0.02±0.00 |
0.07±0.00b |
||
75 |
0.16±0.00 |
0.16±0.00 |
0.65±0.02 |
0.71±0.02 |
0.03±0.01 |
0.04±0.00 |
||
100 |
0.17±0.01 |
0.11±0.00a |
0.69±0.02 |
0.61±0.02 |
0.03±0.00 |
0.05±0.01 |
||
125 |
0.37±0.01 |
0.12±0.02c |
0.59±0.05 |
0.53±0.02 |
0.05±0.00 |
0.05±0.01 |
||
150 |
0.29±0.02 |
0.14±0.01b |
0.73±0.02 |
0.64±0.02 |
0.02±0.00 |
0.04±0.00 |
||
175 |
0.16±0.02 |
0.15±0.01 |
0.53±0.06 |
0.55±0.05 |
0.03±0.01 |
0.03±0.01 |
||
200 |
0.42±0.03 |
0.17±0.01b |
1.13±0.02 |
0.78±0.05b |
0.03±0.00 |
0.05±0.02 |
||
Table 5 Alteration in the various biochemical parameters in the liver of tumor bearing mice treated with different doses of Croton caudatus ethanol extract alone or in combination with 6 Gy of γ-irradiation
Table 6 Alteration in the various biochemical parameters in the kidney of tumor bearing mice treated with different doses of ethanol extract CCE alone or in combination with 6 Gy of γ-irradiation.
p < a = 0.05, b = 0.01, c = 0.001, d = 0.0001, no symbols = not significant when compared to Sham-irradiation group. CCE (Croton caudatus extract). Animals were sacrificed at 10 days.
Ionizing radiations and chemotherapy are important treatment modalities to treat cancer and they have helped the patients to recover from the disease. Despite increased survival and cure rates these modalities increase toxicity in the normal tissues and also in the rapidly dividing tissues leading to morbidity and mortality.12 The combination of radiotherapy and chemotherapy leads to higher toxicities.13 The definite therapy to alleviate the toxic effects of radiotherapy and chemotherapy are still not available, therefore it is imperative to attenuate their toxic side effects without reducing the abilities of these regimens to treat tumors. Herbal medicines have been practiced in the world since the advent of human history and their scientific evaluation may be of great interest to combine them with modern therapy. The application of herbal medicines as adjuvant to radiotherapy may attenuate the side effects of radiotherapy and chemotherapy and at the same time sensitize the neoplastic cells to radiation and protect the normal tissue from radiation or chemotherapy-induced adverse toxic side effect.8 Furthermore, the use of herbal medicines may also accelerate the immune surveillance of normal tissues, which are adversely affected during progression of neoplasia. The herbal medicines may also counter the multiple negative effects induced by radiotherapy or chemotherapy or combination of both. More and more individuals who have been diagnosed with cancer or related illnesses chose alternative medicines. They consult holistic practitioners who are trained to cure ailments with vitamins, herbs, nutrition, homeopathy, and acupuncture. Therefore, inclusion of herbal medicines in radiotherapy/chemotherapy regimens may be of great advantage in improving the therapeutic index cancer patients by killing neoplastic cells and reducing radiation toxicity to normal tissues.14
With the increasing popularity of medicinal plants as alternative therapy, it is essential to conduct systematic research on plants to establish the therapeutic claims of traditional medicine and also ensure that the plants are actually safe for human use.37 The safety evaluation can be performed by evaluating the acute toxicity of any unknown substance and LD50.determination is the most appropriate criterion. The LD50 has been used historically to compare the toxicities of compounds relative to their therapeutic doses and it is now well recognized that high precision may not be required to compare toxicities. Our findings have clearly met the objective of the study, where the various extracts of Croton caudatus leaf (CCE) did not show mortality up to a dose of 0.25 g/kg b. wt. for chloroform and ethanol extracts, respectively and 0.15 g/kg b. wt. for aqueous extract after intraperitoneal administration during the entire observation period as per OECD guidelines. According to the chemical labeling and classification of acute systemic toxicity, based on intraperitoneally LD50 values, the crude extracts of CCE belong to class 5 category (LD50 > 2000 mg/kg b. wt.), which comes under the lowest toxicity class.36 The most effective dose was ¼ of LD50 indicating the safety of drug. The study of biochemical parameters revealed an alteration in the normal levels of ALT, AST, ALP, urea, uric acid and creatinine. In addition to the haematological parameters, biochemical variables are of vital importance in the physiopathological evaluation of any pharmacological agent in the animals. Biochemical tests related to the hepatocellular integrity are performed to evaluate liver damage, where determination of serum activities of AST and ALT provides a clear indication of the extent of liver damage by any pharmacological agent since the level of these enzymes are able to discriminate between liver and cardiac injuries.15 In this study, administration of the CCE caused an increase in the AST, ALT, and ALP activities, and also urea, uric acid and creatinine contents immediately after termination of therapy.
It may be difficult to explain the changing mechanism of serum ALT and AST parameters. It is likely that these increases may be related to deleterious changes initiated by radiotherapy in combination with CCE or radiotherapy alone in the cell. It is well known that AST and ALT are synthesized by hepatocytes and alteration is a measure of liver damage.16 The increase in albumin after administration of different doses of CCE or CCE in combination with irradiation may be due to increased toxicity of the drug with increase in administered dose, which is corroborated by the rise in the activities of AST and ALT. This rise in serum AST and ALT activities indicates the liver damage. Although these enzymes are expressed at a higher level in liver, they are also found in other tissues such as kidney, muscle and heart.17The chronic alcoholism, hepatocellular carcinoma and tissue injury has been found to elevate AST and ALT levels in humans.18 Hence, a simultaneous increase in serum AST and ALT levels at the end of first week of radiotherapy may be probably related to γ–radiation-induced liver, heart and epithelial tissue injury.
The present study indicates that different extract of Croton caudatus were practically non- toxic up to 150 mg/kg b. wt. and the LD50 was 350mg for aqueous and 500 mg and 650 mg for chloroform and ethanol extracts, respectively. The combination treatment has increased the activities of AST, ALT and ALP whereas albumin, total proteins, and total bilirubin remained unaltered in livers of tumorized mice. The kidney of tumorzied mice showed depletion in the urea, uric acid and creatinine contents. The alteration in the biochemical profile by combination treatment may indicate the therapeutic effect on tumor.
All animal procedures were carried out in accordance with the Institutional Animal Ethics Committee of Regional Institute of Medical Sciences, Imphal (Protocol date: 26th November, 2012) conformed to the guidelines set by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA, Chennai, India), World Health Organization, Geneva, Switzerland, INSA (Indian National Science Academy, New Delhi, India) and the “Guide for the care and use of Laboratory Animals” (National Academy of Sciences, USA, 2010).
The authors would like to thank Dr. Jeremy L. Pantu, MD. DM, Head of Department , Medical Oncology, Mizoram State Cancer Hospital, Aizawl, Mizoram and his associates Dr. Khammanpau, Mr. Lallawmzuala, M. Sc. (Physics), medical Physicist, Marilyn Lalzirpuii, Lalrintluangi, Lalhmangaiha and Malsawmkima Pachuau for providing necessary irradiation facilities during the course of this study and dosimetry. The authors are thankful to the University Grants Commission for financial support vide Grant Nos. F.4–3/2007(BSR)/11–116/2008(BSR), and F.4–10/2010(BSR) and Department of Biotechnology vide Grant No. BT/60/NE/TBP/2011), Government of India, New Delhi to carry out this study.
The author declares that they have no competing interests.

©2018 Shantabi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.