Open Access Journal of
eISSN: 2576-4578


Background: Autism and Autism spectrum disorders (ASD) are neurodevelopmental psychiatric disorders characterized by impairments of language, communication, social interaction, stereotype and repetitive behavior. Qualitative impairments in communication such as abnormal language and poor interactive communication skills are fundamental to the diagnosis of autism. Chromosome-engineered Paternal duplicated mouse (PatD/+) pups showed some characteristic features of autism including abnormal USVs at developmental postnatal age when compared with wild type mouse pups.
Objective: To study the effect of an selective 5-HT2CR agonist (WAY161503) on USVs production in paternal duplicated pups and area of brain involve on it
Methods: On the day of experiment pups were kept into the home cage with their dam and transported into the experimental room; left undisturbed for 1 hour. Pups were injected (IP) either with drug or vehicle and put back into the home cage. Thirty minutes later each pup was placed on a metal plate inside the sound proof chamber, where temperature was maintained at 24°C by circulating water through a reservoir below the plate. Ultrasonic sound produced by the pups was recorded for 5 minutes. Genotyping was performed to determine the Paternal duplicated and wild type pups. Quantitative measurement of C-fos was done at different brain region by immunohistochemistry. C-fos is extensively used as a marker of neural activation in brain.
Results: At 1 mg/kg 5HT2C receptor agonist showed changes in USVs in wild type pups compared to vehicle (33±22 vs116±87, P≤ 0.001. No significant difference was observed in case of antagonist ((110±74 vs 70± 26, P= 0.058). No significant difference was observed in alteration of USVs in paternal duplicated pups compared to vehicle group in response to agonist and antagonist (194±114 vs 249±122, P=0.22; 74±62 vs 88±75, P=0.49 respectively). At retroambiguus nucleus brain region, c-fos expression was significantly expressed in paternal duplicated pups compared with wild type.
Conclusion: Reduced USVs in response to 5-HT2CR agonist & increased neuronal activation of retroambiguus (NRA) brain region in paternal duplicated pups reflects the involvement of 5-HT2CR and its phenotypes relevant to the treatment symptoms of autism.
Keywords: chromosome, genotyping, immunohistochemistry, 5-HT2CR, agonist, neuronal activation
ASD, autism and autism spectrum disorders; PatD, paternal duplicated; US, ultrasonic sound; USV, ultrasonic vocalization; PBS, phosphate buffered saline; NGS, normal goat serum; NRA, nucleus retroambiguus; PAG, periaqueductal gray
Autism and Autism spectrum disorders (ASD) are neurodevelopmental psychiatric disorders characterized by impairments of language, communication, social interaction, stereotype and repetitive behavior.1,2 Twin studies have shown that the narrow as well as broad phenotypes of ASD are strongly associated with genetic component.3 Etiology of the disease is unknown but it is thought to involve an interaction between multiple genes and environment factors. Diagnosis can be made by three years of age based on the presence or absence of specific behaviors that are used as diagnosis criteria. Evidence suggested that language disorder is associated in patient with autism. Knockout studies in mice have shown that mutation of certain type gene may cause the impairment of language in autistic patient but still neural mechanism is not known. In recent years several autistic mice model has been developed to investigate the actual cause of autism and ASD. Alteration of ultrasonic vocalization (USV) is frequently reported by using different model of autistic model mice, suggesting that altered USVs is a reminiscent of language impairment in humans.4–6 Infant mice, like the young of all mammalian species including humans, exhibit distress like reactions when they are separated from their dam and littermates.7,8 Their distress is signaled by the emission of 30-80 kHz USVs that can be heard by the dam and trigger their retrieval.9 Other neonatal rodents including rats, hamster and gerbils also emit maternal separation induced USVs.8,10 Drugs that reduce the anxiety in humans attenuate the emission of USVs in rat pups, leading to the use of these reactions as a screen for potentially anxiolytic compounds.14 Some studies have suggested the involvement of 5-HT2CR in anxiety like behaviour and mood disorder in mice.11–13
To date 14 types of serotonin receptors have been reported. Among these the serotonin 2C receptor (5-HT2CR) is functionally important within the limbic corticostriatal circuits which are involved in psychiatric and neurological disorders including anxiety, depression, drug addiction, obesity and schizophrenia. The limbic-corticostriatal circuit functionally connects the prefrontal cortex ventral striatum, amygdala, hippocampus, and other nuclei. Interestingly, 5-HT2CR agonist and antagonist have modulatory effects on USVs production in rat pups.14,15 Chromosome-engineered (15q11–q13) Paternal duplicated mouse (PatD/+) pups had shown some characteristic features of autism including abnormal USVs at developmental postnatal age when compared with wild type mouse pups. Furthermore 5-HT2CR RNA editing data suggested the alteration of 5-HT2CR signaling pathways. In order to investigate the role of 5-HT2CR on USVs production in paternal duplicated pups we hypothesized that alteration of seroternergic system causes abnormal USVs in paternal duplicated pups at developmental stage. To test this hypothesis we examined the effect of a selective 5-HT2CR agonist (WAY161503) on USVs production in paternal duplicated pups. To further find out the brain regions which potentially correspond to USVs production, we have screened the expression c-fos throughout the brain by immunohistochemistry. c-fos is extensively used as a marker of neural activation in brain in response to various types of stress.16,17
Animals
Experiments were conducted on paternal duplicated and wild type pups at postnatal day 7. All pups were housed with their dam and littermates in a plastic cage containing wood shavings as bedding until the day of experiment. All the mice were maintained in animal room at controlled room temperature and standard light-dark condition (12:12h light–dark). Food and water were available at libitum. On the day of experiment pups were labeled and measured their body weight. Before the experiment pups were kept in an experimental room with their dam for 1 h for adaptation in a new environment. Pups were then divided into vehicle and drug group. Data were taken so that each group contains 14 numbers of pups. The experiments protocol was carried out in accordance with the guidelines of the Animal Welfare Committee of Osaka Bioscience Institute.
Ultrasonic vocalization
On the day of experiment pups were transported into the laboratory. After Labeling and measuring the weight, pups were again kept into the home cage with their dam and transported into the experimental room; left undisturbed for 1 h. Pups were injected (IP) either with drug or vehicle and put back into the home cage. Thirty minutes later each pup was placed on a metal plate inside the sound proof chamber, where temperature was maintained at 24°C by circulating water through a reservoir below the plate. Pups were covered by a hollow metal cylinder and microphone was adjusted 5 cm apart from the plate through the top of the cylinder. Ultrasonic sound produced by the pups was then recorded for 5 min (USV recording). More than 20 ms duration was considered as a one call. At the end of the test the pups were returned to their home cage. Genotyping was performed to determine the Paternal duplicated and wild type pups.
Drug Treatment
Selective Serotonin 2C receptor agonist (161503) and antagonist ( 242084) was dissolved in a deionized water and solution containing 0.9% saline, 8% hydroxypropyal-beta cyclodextrin, 25 mm citric acid respectively. Pups were received intraperitoneally selective serotonin 2C receptor agonist and antagonist 30 min prior to experiment. In case of vehicle group saline was injected. Pups were injected the same volume (10ml/kg) but different concentration of drug (0mg/kg, 0.3mg/kg, 1mg/kg and 3mg/kg)
C-fos immunohistochemistry
C-fos expression was determined by immunohistochemistry. On the postnatal day 7 of both genotype of pups (PatD/+ & WT) were injected with saline prior to 30 min of USV recording. After 5 minutes of USV recording, Pups were separated from their dam for 2 hour to achieve the peak cfos expression.18,19 Pups were intraperitoneally injected with an overdose of Nembutal, expose the heart by opening of chest and perfuse with cold PBS. PH 8.0, followed by cold 4% Para-formaldehyde in phosphate buffered saline (PBS). The brains were removed and post fixed with 4% PFA for overnight followed by 30% sucrose solution for cry protection. The brains were embedded with 1:1 dilution of 30% sucrose and PBS, frozen and stored in -80°C until cutting the sections. OCT embedded pups brain was sectioned in a freezing microtome at 30μm and sections were collected in every 300μm of distance from whole brain by attaching on a mash coated glass slide. All the slides were dip into the PBS to remove the OCT from the sections, air dried overnight and stored at -80oC until start the experiment.20
On the day of cfos experiment sections were removed from the fridge and start the procedure after came down at room temperature. The sections were washed twice (2x5 min) in PBS solution, incubated in 0.3% H2O2 solution for 30min to block the activity of endogenous peroxidase, then washed again in PBS solution (2x5min) and incubated in a 3% normal goat serum (NGS) blocking solution (PBS/3% NGS/0.3% Tritonx100) for 2 hour. Subsequently sections were incubated in rabbit polyclonal cfos antibody (IgG) diluted (PBS/0.1% NaN3/0.3% Tritonx100) at 1:20000 (oncogene) for overnight at room temperature, washed in PBST (PBS/0.3% Tritonx100) solution (6x10 min),then incubated with biotynilated anti rabbit IgGs for two hour, washed in PBST(3x10min) and incubated with Avidine biotine peroxidase complex (Vector laboratories, CA) for 1 hour. Finally, after being washed in PBST (2x10min) sections were immunoreacted with a solution containing PBS, 0.04% diaminobenzidine hydrochloride (DAB) and 0.02% H2O2 for 20min and washed in PBS (1x5min). The slides were then dehydrated by serial alcohol rinsing. Cleared in xylene and cover-slipped with mount quick. C-fos immune reactivity was assessed by using Olympus microscope at 20x magnification, which is equipped with a camera to take the photograph of the sections. Area of the brain sections were determined by matching with paxinos atlas.
Effect of 5-HT2C receptor agonist and antagonist on USVs production
First we optimized the concentration of serotonin 2C receptor agonist and antagonist drug (0mg/kg, 0.3mg/kg, 1 mg/kg and 3mg/kg) by using wild type pups shown Figure 1. In wild type agonist (WAY 161503) group, at 0.3mg/kg USVs production was same with vehicle group (59±42 vs 62±38, P=0.86). At 1 mg/kg drug dose, USVs production was significantly reduced compared with vehicle group (33±22 vs 116±87, P≤0.001). At dose 3 mg/kg, USVs production was reduced in drug group relative to vehicle group but statistically not significant (58±71 vs 89±78, P=0.22). Similarly in wild type antagonist (SB 242084) group at dose 0.3mg/kg USVs production was increased as compared with vehicle group but statistically not significant (106±86 vs 72±83, P=0.29). At dose 1 mg/kg also no significant difference of USVs production was observed in between two groups. At dose 3mg/kg, USVs production was increased as compared with vehicle group (110±74 vs 70±26, P=0.05).
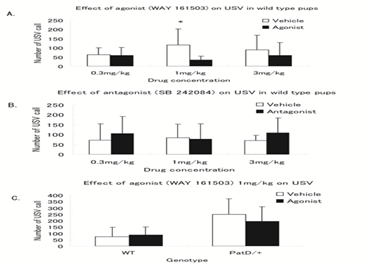
Figure 1 (A) Effect of agonist (WAY 161503) at concentration 0.3 mg/kg and 3 mg/kg on USV in wild type pups. USVs were significantly reduced at 1 mg/kg dose in drug group compared to vehicle group. (P<0.001,*). (B) Effect of antagonist (SB 242084 at different concentration in wild type. Trend of USVs call were increased in drug group at concentration 0.3 mg/kg and 3 mg/kg dose in drug group compared to vehicle group. (C) Effect of agonist (WAY 161503) 1 mg/kg concentration on USVs call in wild type and paternal duplicated pups. In both genotypes trend of USVs call were reduced in drug group compared to vehicle group but statistically not significant.
Since only agonist at 1 mg/kg had effect on USVs production, we never investigate the effect of antagonist on USV production in patD/+ pups. In normal condition PatD/+ pups produced more USVs than WT which is consistent with previous experiment in our laboratory. USV in PatD/+ pups were reduced compared with vehicle group but the difference was not significant (194±114 vs 249±122, P=0.22). Similarly in wild type from the same littermates, at dose 1 mg/kg no significant USVs production was detected between drug and vehicle group (74±62 vs 88±75, P=0.49)
We performed the c-fos immunohistochemistry to investigate the neuronal activation circuit in response to maternal separation in paternal duplicated pups. At the beginning of experiment, we checked the reproducibility of the test by treating with acetic acid in wild type adult mice. The expression of c-fos was detected in several brain regions as shown in Figure 2.
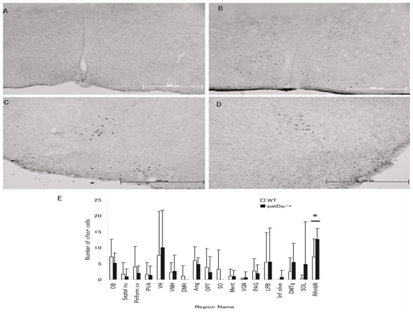
Figure 2 Expression of c-fos at low magnification in different regions of mice brain (A) and (B) Medial preoptic area of wild type mice treated with saline and acetic acid respectively. (C) & (D) Retro ambiguous nucleus (RAmbN) of wild type and paternal duplicated pups respectively at postnatal day 7 in response to 2 h maternal separation stress. (E) Quantitative analysis of the density of Fos like immunoreactive cells in the different regions of the pups brain.
At postnatal day 7, several region of the brain expressed c-fos in both paternal duplicated and wild type pups but the expression of c-fos was not frequently detected in some region, shown in Figure 2. In most of the region quantitative analysis of c-fos data was not statistically significant difference in paternal duplicated compared with wild type. Interestingly in retroambiguus nucleus, c-fos expression was significantly expressed in paternal duplicated pups compared with wild type, P≤0.03.
USVs were elicited in mouse pups at middle period of developmental postnatal age but previously our laboratory reported the marked production of USVs at postnatal day 7 in paternal duplicated pups compared to wild type. We again confirmed the previous USVs data and investigated the role of seroternergic system to production of USVs by using highly selective 5-HT2C agonist and antagonist. At dose 1 mg/kg of agonist (WAY 161503) production of USVs were significantly reduced compared to vehicle group in wild type, similar to previous report.15 In other concentration (0.3mg/kg, 3mg/kg) production of USVs were slightly reduced but statistically not significant. Dose dependent effect of 5-HT2C agonist on USV was documented. In most cases these reductions of USV caused by 5-HT2C receptor agonists were not selective.
The reason we have selected the WAY 161503 is highly selective (10 fold) to 5HT2CR over 5HT2AR. 5HT2C antagonist increased the USVs production; we could get the similar trend of results at doses 0.3mg/kg, 1mg/kg and 3mg/kg as compared to vehicle group. Again the effect of antagonist is dose dependent so it may have effect in higher concentration than we used in this experiment. In paternal duplicated pups we applied a 1mg/kg concentration of agonist to see the responsiveness of 5-HT2CR. At this concentration USVs production was reduced as previously reported but it was not significant difference compared vehicle group. Due to highly production of USV insensitive to low concentration of drug or it may be due to high variation of USVs within a group after injecting a drug (WAY 161503) and third we may require administering a high dose of drug.
In second experiment we checked a neuronal activation circuit by using c-fos expression in paternal duplicated pups. In response to maternal separation stress several region of brain express c-fos, a marker of neuronal activation but the appearance of c-fos signal was not consistent in some region. It may be due to the high individuality of USVs production in paternal duplicated pups. Nucleus retroambiguus (NRA) significantly expressed the c-fos compared to wild type, which may reflect the involvement of RAmbN in production of abnormal USVs. The nucleus retroambiguus (NRA) of the caudal medulla is a relay nucleus by which neurons of the mesencephalic periaqueductal gray (PAG) reach motoneurons of pharynx, larynx, soft palate, intercostals and abdominal muscles, and several muscles of the hind limbs. These PAG-NRA-motor neuronal projections are thought to play a role in survival behaviors, such as vocalization and mating behavior. The activated region of the brain which has been shown in Figure 2, May involved in the abnormal production USVs.
5-HT2C agonist reduced the USVs in paternal duplicated pups which reflects the involvement of 5-HT2CR. Neuronal activation at nucleus retroambiguus (NRA) further supported the present findings to its phenotypes relevant to the treatment symptoms of autism.
Financially this work was supported by Takeda Science Foundation, Juso, Osaka, Japan.
All authors have contributed to the work.
All experiments were approved by Animal Welfare Committee of Osaka Bioscience Institute.
We would also like to thanks to all laboratory staff for directly or indirectly contribution to conduct this work.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.