Open Access Journal of
eISSN: 2576-4578


Mini Review Volume 1 Issue 2
1Bahra Institute of Pharmacy, Rayat Group of Institutions, Patiala?147001, Punjab, India
2GVM, College of Pharmacy, Haryana, India
3Department of Pharmaceutical Sciences and Technology, Maharaja Ranjit Singh Punjab Technical University, Bathinda, Punjab, India
Correspondence: Preet Amol Singh, Department of Pharmaceutical Sciences and Technology, Maharaja Ranjit Singh Punjab Technical University, Bathinda, Punjab, India, Pin code 151001, Tel +91-8437000234
Received: October 01, 2017 | Published: October 18, 2017
Citation: Boparai A, Niazi J, Bajwa N, et al. Betulin a pentacyclic tri–terpenoid: an hour to rethink the compound. Open Access J Trans Med Res. 2017;1(2):53-59 DOI: 10.15406/oajtmr.2017.01.00012
Betulin a pentacyclic triterpenoid member of lupane family occurs widely in numerous plants. Betulin, unlike most other constituents is easily isolated that it can be utilized for various pharmacological actions. It is interesting to know that betulin can be easily extracted from bark, stem, leaves, flower, roots etc. of plant. This review summarizes plethora of reliable pharmacological activities like anti–inflammatory, anti–ulcer, anti–diabetic, anti–bacterial, anti–microbial, anti–malarial, anti–viral, anti hyperlipidaemic, anti–cancer and anti HIV exhibited by betulin and its derivatives. For this it can be utilized in herbal as well as synthetic pharmaceutical industries because of its promising efficacy and low levels of toxicity. Although betulin possess a wide range of pharmacological activities, there is still lack of awareness of its proper utilization in the field of medicine. So, need of the hour is to refocus on naturally occurring betulin and its derivatives to avoid side effects caused by synthetic compounds utilized in treating various ailments. Therefore, the aim of the present review is to re–explore the potential of betulin, as an alternative to the compounds possessing higher side effects.
Keywords: betulin, pentacyclic triterpenoid, betulinic acid, lupine, pharmacological activities
Mother earth, a bionetwork enriched with plethora of remarkable plants holding numerous beneficial chemical compounds, which play an integral role in maintaining the lives stealth and safe. Betulin lup20 (29)–ene–3ß, 28–diol, a pentacyclictriterpene alcohol with a lupane skeleton is one of such chemical compounds contributing towards the advancement in field of medicine.1–3 It is obtained from outer bark of Birch trees.4–6 As described in Figure 1: Betulin has a pentacyclic ring structure and hydroxyl groups in positions C3 and C28.7–9 Betulin in presence of acid agents undergoes rearrangement to form allobetulin.10 It is acknowledged that plants with lupine series are helpful in curing various diseases for this one relies on betulin which on conversion tobetulinic acid, the alcohol group replaced by a carboxylic acid group has more biological activity.2,7,10,11 The alcohol group cannot join with stationary phase because two groups are located on opposite sides of compound.1 Betulinic acid, the more bioactive compound exhibits choleritic, antihelmintic, powerful prophylactic, anti–HIV, antimutageneagent, antiviral, anti–fungal, anti–leukemia, anti–leishmanial, anti–inflammatory, immunomodulator.7,12–15
Adding on it acts as anti–parasitic against Plasmodium falciparum and Trypanosomabruceirhodesiense, anti–microbial, anti–obesity by improving the lipid profile, also stabilizes atherosclerotic plaques.15,16–20 Betulin is also used as plaster for sterilization of wounds, acts as liver protectant in chronic hepatitis therapy, antirachitic, antitrypanosomal also supports apoptosis i.e. self–destruction of tumor cells.2,7,21,22 As betulin is found in various other plants so people of vernacular regions take numerous benefits depending on the necessity. Infusion of red alder is used in lymphatic disorders and tuberculosis. Native Americans use it to mediate insect bites, poison oak, digestive tract infections and skin inflammation.23–26 Rather than possessing pharmacological activities, due to elegant bark of Birch trees they are considered choice of trees for landscape too. The substantial Birch trees grows well in all soils due to which they are used as screen or window break. Moreover, due to hard bark they are also used in carpentry and aquatic industry. It is also used in cosmetic products. The birch bark extract may be used in hair conditioners and shampoos.27–29
Standard betulin has weak water solubility, thus it requires modification for better cellular uptake and desired activity however its derivatives like betulin diacetate (BDA) and betulin dipropionate (BDP) possess greater water solubility as compared to betulin.32,33 Therefore, betulin still remains relevant in synthesizing compounds with higher solubility pattern that are considered more biologically active (Table 1) (Figure 1).34
1. |
Chemical formula |
C30H50O2 |
2. |
Synonyms |
Betulin, |
3. |
Appearance |
White, crystalline powder |
4. |
Molar mass |
442.7 g/mol |
5. |
Melting point |
256-257 °C (lit.) |
6. |
Solubility |
Alcohol, chloroform, benzene |
7. |
Heat capacity |
80-350K |
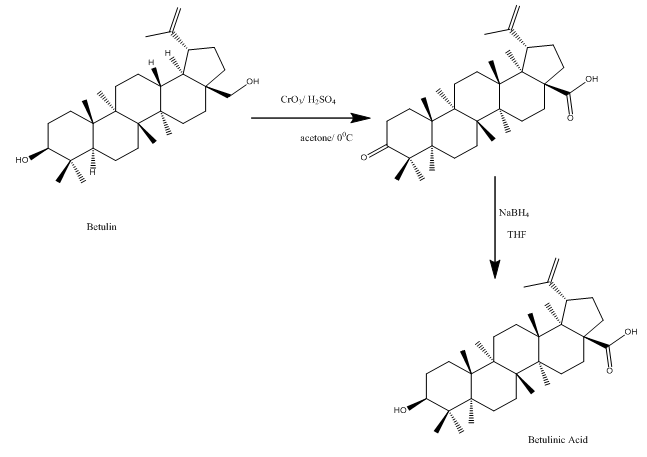
Figure 1 Represents the preparation of betulinic acid from betulin 7-11.
Betulin has long been explored by research scientist because of the fact that it has anticancer properties35,36 and its derivative Betulinic acid is favored due to its anti–HIV activity.12,6,37 As mentioned in Figure 1: betulin has three most prominent positions where chemical modifications can be easily accomplished, namely primary hydroxyl group at position C–28, secondary hydroxyl group at position C–3, and alkene moiety at position C–20.The bio–chemical modifications at positions C–28 of the parent structure of betulin produces betulinic acid.12,27,38–41 The chief source of Betulin is bark of Birch trees, belonging to family Betulaceae the family of flowering plants. Mostly placed in order Fagales, it can also be placed in order Betulales. The sub–families include Betuloideae genera Betula (birch), Alnus (alder) and Coryloideaegenera Carpinus (hornbeam), Corylus (hazel), Ostrya and Ostryopsis.42–44 Betulin, a pentacyclictriterpenoid is derived from linear hydrocarbon squalene.31 Triterpenes have three main classes oleane, ursane and lupane triterpenes. Lupane family comprises betulin, betulinic acid, lupeol.6,45 Triterpenes are used as traditional herbal medicine and the esters of betulin and fatty acids are used in the production of cosmetics and as plasticizers for PVC (Table 2).31,46
S. No |
Name of Plant |
Family of Plant |
Part of Plant |
Reference |
1 |
Betula pumila |
Betulaceae |
Bark |
[47] |
2 |
Betula pendula(silver birch) |
Betulaceae |
Bark |
[35,47-51] |
3 |
Betula jacquemontii |
Betulaceae |
Bark |
[47] |
4 |
Betula pubescens |
Betulaceae |
Bark |
[29,47] |
5 |
Betula platphylla |
Betulaceae |
Bark |
[29,47] |
6 |
Betula papyrifera(paper birch) |
Betulaceae |
Bark |
[12,30,47,52] |
7 |
Betula nana |
Betulaceae |
Bark |
[47,53] |
8 |
Betula nigra |
Betulaceae |
Bark |
[47] |
9 |
Betula lente |
Betulaceae |
Bark |
[47,54] |
10 |
Betula alba |
Betulaceae |
Bark |
[29,49] |
11 |
Betula occidentalis |
Betulaceae |
Bark |
[55] |
12 |
Platanus acerifolia |
Platanaceae |
Bark |
[56] |
13 |
Sambucus nigra |
Adoxaaceae |
Bark |
[57] |
14 |
Olea europeae |
Oleaceae |
Bark |
[58] |
15 |
Aldus subcordata |
Betulaceae |
Bark |
[59,60] |
16 |
Ziziphus jujube |
Rhamnaceae |
Bark |
[59,61] |
17 |
Atractylis carduus |
Asteraceae |
Aerial parts of plant |
[59,62] |
18 |
Platanus hypbrida |
Platanaceae |
Bark |
[59,63,64] |
19 |
Platanus hyspanica |
Platanaceae |
Bark |
[48] |
20 |
Nerium oleander |
Apocynaceae |
Leaves |
[8] |
21 |
Dillenia indica |
Dilleniaceae |
Stem bark |
[2,12] |
22 |
Tectona grandis |
Verbenaceae |
Stem bark |
[2] |
23 |
Alangium salvifolium |
Cornaceae |
Seeds |
[65-67] |
24 |
Alstonia scholaris |
Apocynaceae |
Stem bark |
[68] |
25 |
Cornus macrophylla |
Cornaceae |
Stem bark |
[66-69] |
26 |
Plumeria obtusa |
Apocynacae |
Leaves |
[70-72] |
27 |
Astercantha longifolia |
Acanthaceae |
Aerial parts of plant |
[73] |
28 |
Aerva lanata |
Amaranthaceae |
Flower, leaf |
[74] |
29 |
Quercus suber |
Fagaceae |
Bark |
[75,76] |
30 |
Acacia mellifera |
Fabaceae |
Bark |
[77] |
31 |
Celtis philippinensis |
Cannabaceae |
Twigs |
[78,79] |
32 |
Coccoloba acrostichoides |
Polygonaceae |
Aerial parts |
[80,81] |
33 |
Anemone raddeana |
Ranunculaceae |
Roots |
[82.83] |
34 |
Diospyros leucomelas |
Ebenaceae |
Leaves |
[29,84] |
35 |
Ziziphus vulgaris |
Rhamnacae |
Seeds |
[29,84] |
36 |
Trochodendron aralioides |
Trochodendraceae |
Bark |
[29,84] |
37 |
Torenia concolor |
Scrophulariaceae |
Flower |
[85-87] |
38 |
Belamcanda chinensis |
Iridaceae |
Root |
[88] |
39 |
Chaenomeles sinensis |
Rosaceae |
Fruit |
[89,90] |
40 |
Cyrtomium fortumei |
Dryopteridaceae |
Rhizomes |
[91] |
Table 2 List of plants possessing betulin.
Betulin, as we all known till now possess vast pharmacological properties including choleritic, antihelmintic, powerful prophylactic, anti–HIV, antimutagene agent, antiviral, anti–fungal, anti–leukemia, anti–leishmanial, anti–inflammatory, immunemodulator activities. The latest research suggests some of the major pharmacological properties as discussed below.
Anti–inflammatory and Anti–ulcer activity
Inflammation is a physiological process which involves pain as a secondary process and its hallmarks include swelling, redness, pain and fever.92 Bernard et al.93 determined that Betulin and betulinic acid were found to inhibit phospholipaseA2 activity at 5M concentrations by 30% and 40% respectively.93 It has also been demonstrated to exhibit inhibitory effects on nitric oxide (NO) and prostaglandin E2 production in mouse macrophages.94 According to the latest research done by Singh et al.2 stem bark extract of Dillenia indica f. elongata (Miq.) Miq.showed significant (P<0.01) anti–inflammatory activity in formalin and carrageen an induced inflammation models.12 Moreover, Betulonic acid exhibited antiulcer action exceeding that of Venter preparation for the models if affection of mucous coat of stomach in rats caused by indomethacin and aspirin with the dose of 50mg/kg.95
Anti–diabetic
Diabetes is a metabolic disorder associated with abnormalities in insulin production or secretion along with modifications in carbohydrate, fat and protein metabolism.96,97 In accordance to, Riya et al.,74 determined the presence of betulin, alpha amyrin and beta sitosterol in flower, leaf and roots of Aerva lanata.7,98 However, there are reports that betulin is useful in treatment of diabetes.99,100 As 70% ethanolic extract (ALE) for 21 days in STZ–induced diabetic rat demonstrated that ALE was successful in refining postprandial hyperglycemia in sucrose–loaded normal and STZ diabetic rats, through its promising alpha glucosidase inhibitory potential.101,102 Agarwal et al.101 reported that the alkaloid–enriched fraction of root of Aervalanata possesses anti-hyperglycemic potential in streptozotocin–nicotinamide–induced type II diabetic rats.103
Anti–bacterial and anti–microbial activity
Bacterial infections alone are the cause of around two million deaths globally and it is found that bacterial pathogens probably infect more than one–third of the population around the world.104
In accordance to Valterová et al.,103 the antibacterial activity of C–3 substituted derivatives of betulin with respect to a number of bacteria (Staphylococcus aureus, Staphylococcus faecalis and Staphylococcus beta haemolyticus) was depicted.105 Furthermore, Hess et al.,104 concluded that Betulinic acid has been found to be inactive against Staphylococcus aureus, Escherichia coli, Bacillussubtilis and Micrococcus luteus.106
Antimicrobial activity of betulin and its derivatives have been reported against Streptococcus pyogenes with a minimum inhibitory concentration (MIC) of 85µg/mL, and considerable activity has also been observed against other bacteria, i.e. Escherichia coli, Staphylococcus aureus and Enterococcus faecalis.107,108
Anti–malarial activity
Betulin, betulinicacid, ursolic acid and oleanolic acid have also been tested for monitoring antimalarial activity against chloroquine sensitive (T9–96 strain) and resistant (K1 strain) Plasmodium falciparum. It was concluded that betulin was inactive, whereas the others showed moderate activity, betulinicacid being most active in vitro against both strains of P. falciparum at IC50 values 19.6g/mL (K1) and 25.9g/mL (T9–96) respectively.109 But, in vivo experiments with the NK65 (P. Berghei) model of malaria revealed that betulic acid turned out to be inactive and even toxic at the dose of 250mg/kg per day.110
Anti–viral activity
According to Karachurina et al.,109 Betulinbishemiphthalate and betulin dinicotinate stimulate the production of antibody–forming cells in mouse spleen 1.3 and 1.8 times more actively in comparison with the reference.111 Adding on, the indicated compounds prevent death of animals from acute radiation sickness. However, Kanamoto et al.110 and Baltina et al.111 studied anti–viral activity of betulin, betulinic acid and its derivatives against influenza A, herpes simplex type 1 (HSV–1), influenza FPV/Rostock and ECHO–6 enterovirus. Betulin and betulinicacid were inactive against influenza FPV/Rostock virus on the other hand betulonic acid 3 showed a weak antiviral activity.112,113
Anti–hyperlipidaemic activity
Tang et al.16 identified a small–molecule inhibitor of SREBP, betulin, by compound screening. Where SREBP is a major transcription factor that controls the biosynthesis of cholesterol, fatty acid, and triglyceride.114 Betulin inhibits SREBP by binding SCAP and making the interaction between SCAP and Insig easier, which leads to the ER–retention of SREBP. Betulin down regulates the genes in cholesterol and fatty acid biosynthesis and decreases the content of cellular lipids, enhances insulin sensitivity, and reduces the development of atherosclerotic plaques.16
Anti–cancer activities
According to the latest research done by Bębenek et al.114 Betulin and its semisynthetic derivatives possesses cytotoxic activity toward various cancer cell lines. Experimentation for the antiproliferative activity in vitro against T47D breast cancer, CCRF/CEM leukemia, HL–60 promyelocyticleukemia, SW707 colorectal, murine P388 leukemia, as well as BALB3T3 normal fibroblasts cell lines was done by using betulin and its derivatives. It was discovered that that the derivative of betulin with a propynoyl group at C–28 position, has strong cytotoxic effects against human leukemia (CCRF/CEM) and murine leukemia (P388) cancer cells.115
Anti–HIV activities
Hashimoto et al.115 researched that betulin and 3, 28–diacetylbetulin are inactive as anti–HIV agents which confirm the importance of the presence of carboxylic group at C–28.116 However, betulinic acid and its derivatives have been discovered as a new class of compounds that seem to act as immunomodulator and protect the cells invitro from attack by the HIV virus.117 Furthermore, synthetic betulinicacid derivatives, especially 3–alkylamido–3–deoxy–betulinic acid derivatives, inhibit the life cycle of the virus in the infected cells in its early phase hence; defend the surrounding cells from HIV proliferation.118 Also, one must take into consideration that anti–HIV activity increases in amides and peptides of betulic and betulonic acids.119,120
It is commendable that betulin is found in 200 different types of plants indefinitely distributed across the plantae kingdom and owes to diverse pharmacological activities. But despite of its easy and free availability in nature, betulin and its derivatives are still not empathized in pharmaceutical industries. The good side of picture is that the isolation of betulin is not a tough job and need not require complex analytical techniques. The tool of biotechnology can further be applied to gain maximum pharmaceutical advantages of betulin. Due to the fact that it is a compound obtained from plant source and possess vast significant pharmacological properties it can be utilized in herbal pharmaceutical industries with a new concept of nano–medicine. Having minimal side effects gives betulin an edge over other compounds and plant extracts containing betulin are also of equal importance for possessing signified efficacy resulting in decreased level of toxicity. At last, it seems that betulin requires our re–attention so that with the help of growing analytical techniques we can produce its new derivatives which could be a boon to society for treating various ailments.
The authors are very thankful to the Dr. Charanjit Singh for sparing his valuable time for this article.
The author declares no conflict of interest.

©2017 Boparai, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.