Open Access Journal of
eISSN: 2575-9086


Research Article Volume 7 Issue 1
1Environmental Microbiology Laboratory, Chemical and Biological Research Institute Ed B3 C.U., University City. Universidad Michoacana de San Nicolás de Hidalgo, Fco J Mujica S/N, Col Felicitas del Rio, ZP 58030, Morelia, Michoacan, México
2Departamento de Parasitología, Universidad Autónoma Agraria Antonio Narro #1923, Buenavista, Saltillo 25315, Coahuila, México
3FES, Zaragoza, Universidad Nacional Autónoma de México, Av. Guetato 66, Ejercito de Oriente, INDECO, ISSSTE, Iztapalapa, 09320, Ciudad de México. CDMX, México
4Chemical Engineering, University City. Universidad Michoacana de San Nicolás de Hidalgo, Fco J Mujica S/N, Col Felicitas del Rio, ZP 58030, Morelia, Michoacan, México
Correspondence: Juan Manuel Sánchez-Yáñez, Environmental Microbiology Laboratory, Chemical and Biological Research Institute Ed B3 C.U., University City. Universidad Michoacana de San Nicolás de Hidalgo, Fco J Mujica S/N, Col Felicitas del Rio, ZP 58030, Morelia, Michoacan, México
Received: June 08, 2024 | Published: June 25, 2024
Citation: Sánchez-Yáñez JM, de la Cruz JLI, Gallegos-Morales G, et al. Xanthobacter autotrophicus and endophytic yeasts preventing greenhouse gases in the growth of Phaseolus vulgaris. Open Access J Sci. 2024;7(1):121-127. DOI: 10.15406/oajs.2024.07.00224
Currently, in agricultural production, to ensure that it is not a source of greenhouse gases, without affecting the healthy growth of Phaseolus vulgaris, it is necessary to apply NH4NO3 at 70% since previous studies indicate that values of 100 to 80% are uptake by the plant (data not showed) and generate N2O in addition to contaminating surface and underground water. An alternative solution is to apply NH4NO3 70% and inoculate the seeds with microbial consortia that optimize this nitrogen fertilizer. The objective of this research was to analyze the response of P. vulgaris to Pichia norvegensis, Saccharomyces cervesiae and Xanthobacter autotrophicus NH4NO3 at 70%. The experiment was carried out with a randomized block design; the response variables: germination percentage, days of emergence, phenology: plant height (PH), root length (RL) and biomass: aerial/radical fresh weight (AFW/RFW) aerial and radical dry weight (ADW/RDW) of P. vulgaris. All the experimental data were analyzed by ANOVA/Tukey HSD (P<0.05).
The results showed a positive effect of P. norvegensis and X. autotrophicus with 94% germination of P. vulgaris seeds; at seedling stage registered 37.48 cm of PH, 18 cm of RL, 1.96 g of FAW, 1.55 g of RFW, 0.24 g ADW and 0.14 g RDW, all this numerical values statistically were different, compared to 70.7% germination, 28.8 cm PH, 10.66 RL, 0.82 g AFW, 0.29 g RFW, 0.12 g ADW and 0.03 g RDW in P. vulgaris not inoculated with P. norvegensis or X. autotrophicus fed with 100% NH4NO3 , used as relative control (RC). These results support that it is feasible to use interactions between yeasts and endophytic bacteria, such as P. norvegensis and X. autotrophicus to activate and improve the physiological capacity of P. vulgaris root to increase NH4NO3 70% uptake, which prevents the release of greenhouse gases associated with global warming, loss of fertility and contamination of surface and groundwater.
Keywords: soil, chemical fertilizer, endophytic microbes promoting plant growth, climatic change
Healthy growth of Phaseolus vulgaris depends on nitrogen fertilizers such as NH4NO3 in excess generates a loss of soil productivity,1–3 by unbalancing the carbon: nitrogen ratio, it causes changes in microbial diversity, affecting biogeochemical cycles necessary for plants,4,5 excess nitrogen fertilizer generates greenhouse gases6 surface and groundwater pollution.7 An ecological alternative, to regulate nitrogen fertilizer application, is to decrease NH4NO3 to 70% and inoculate seeds of P. vulgaris, with multiple natural endophytes such as: Pichia norvegensis, Saccharomyces cervesiae and the nitrogen-fixing prokaryote Xanthobacter autotrophicus, capable of invading the root tissue, to transform intermediate compounds of plant metabolism, into phytohormones to enhance root hairs for maximum exploration area, in the soil to optimize NH4NO3 to 70%.8–10 The application of bacteria and fungi is common, but not yeasts and endophytic bacteria.11,12 In this regard, Agamy & Alamri,13 analyzed the positive response of Beta vulgaris beer to Sccaromycopsis cataegensis, at a restricted dose of nitrogen fertilizer, with values of aerial dry weight statistically different compared to ADW and RDW in the non-inoculated B. vulgaris. This form of plant biomass enhancement is reported by Ignatova et al, 2015 as they demonstrated that Rhodotorula mucilaginosa, synthesizes phytohormones to induce root formation, and optimize nitrogen fertilizer uptake, that can be enhanced with X. autotrophicus endophyte due to the wide interaction with domestic and forest plant species, so the objective of this research was to analyze the response of P. vulgaris to P. norvegensis, S. cervesiae and X. autotrophicus and NH4NO3 70% preventing global warming due to greenhouse gases.
This research was conducted in the greenhouse of the Environmental Microbiology Laboratory, Research Institute in Chemistry and Biology, Universidad Michoacana de San Nicolás de Hidalgo (UMSNH), Morelia, Michoacán, Mexico, in a greenhouse under the following average microclimatic conditions: 23.2 °C, luminosity of 450 μmol m-2s -1 and 67 % relative humidity. In a non-sterile soil collected in the municipality of Salvador Escalante, Michoacán, Mexico with a silty clay loam texture with 10.44% organic matter, a moderately acid pH of 5.75, the soil was classified according to NOM-021.RECNAT-2000, the soil was sieved with No. 20 mesh, solarized at 70°C/48 h to minimize pests and diseases.
Origin of endophytic yeasts
The isolation of plant growth promoting endophytic yeasts was from the root, stem and leaf of Amarylis belladonna (summer lily), Clivia miniata (clivia), Spanthodea campanulata (african tulip). For this, plant tissues were disinfected with a 3% sodium hypochlorite solution/3 min, washed 6 times with sterile water, then disinfected with 75% ethanol/3 min and washed 6 times with sterile water. The plant organs were cut into 5 cm pieces, macerated with a 0.85% saline solution RomaMR 0.01% detergent (SSD), from there 1.0 mL was taken in yeast malt agar (YMA) (g/L): 3.0 yeast extract, 3.0 malt extract, 10.0 sucrose, 5.0 peptone of casein, 18.0 of bacteriological agar, adjusted to pH 5.5 and 10 mL of ciprofloxacin at 50 mg/mL to eliminate any bacteria, then incubated at 30°C/36 h. of 5.5 to 6; Malt Yeast Extract Agar or MYEA (g/L): malt extract, 3.0; yeast extract, 3.0; peptone, 5.0; glucose, 10.0; 18.0 agar, pH 5.0; Malt Yeast Broth or MYB (g/L): malt extract, 3.0; yeast extract, 3.0; peptone, 5.0; glucose, 10.0, pH at 5.0; Wickerham-1 (g/L): glucose 10, yeast extract, 4.5; peptone, 7.5 and pH 6.5;and Wickerham-2 (g/L): KH2PO4, 0.15; MgSO4H2O, 0.5; NaCI, 0.1; CaCI, 0.1;dextrose, 10.0; NH4CI, 0.5, pH 6.5, 1 mL of ciprofloxacin was added to each culture medium at a concentration of 50 mg/mL (Sánchez-Yañez, 2007).
Identification of plant growth promoting endophytic yeast
Subsequently, all the culture media were inoculated with 500 and 1000 µL, then incubated until growth was observed at 27°C and performed the following biochemical tests: catalase, fermentation of glucose and sucrose, Simmons citrate, P. norvegensis, and S. cerevisiae on yeast malt agar (YMA) with the following chemical composition (g/L): 3 yeast extract, 3 malt extract, 10 sucrose, 5 casein peptone, 17 bacteriological agar, 10 mL of ciprofloxacin was added at a concentration of 50 mg/mL pH 5.5.
X autotrophicus was kindly donation from Department of Chemical and Biology, Harvard University, Cambridge, Ma, USA, X. autotrophicus grew on non-sucrose or nitrates agar with the following chemical composition (g/L): K2HPO4 3.0, MgSO4 .7H2O 0.5, KCl 0.5, FeSO4 0.01, bacteriological agar 18, pH 7.
Molecular identification of endophytic yeast isolates
The 5.8S internal transcribed spacer (ITS) rDNAs of yeast isolates ARDMC1 and S. cerevisiae and P. norvegensis were amplified using the primers ITS-1 (50-TCCGTAGGTGAACCTGCGG-30) and ITS-4 (50-TCCTCCGCTTATTGATATGC-30) according to White et al., 1990. The D1/D2 domain of the 26S rDNA gene was amplified using the primer pair NL1 (50-GCATATCAATAAGCGGAGGAAAAG-30) and NL4 (50-GGTCCGTGTTTCAAGACGG-30). The polymerase chain reaction (PCR) amplification was performed in an Eppendorf Thermocycler according to a protocol14 amplification parameters consisted of an initial denaturation step of 3 minutes at 95◦C, followed by 30 cycles of 95◦C for 30 seconds, primer annealing for 30 seconds at 58◦C, elongation for 1 minute at 72◦C, and final extension of 10 minutes at 72◦C for one cycle. The amplified PCR product was purified and subjected to automated DNA sequencing using a 3130 Genetic Analyzer (Applied Biosystems, Rotkreuz, Switzerland).
Inoculation of seeds of P. vulgaris with endophytic yeast and X. autotrophicus
The P. vulgaris seeds were disinfected with 5% NaClO (sodium hypochlorite) for 5 minutes, then washed 5 times with sterile water, disinfected in 70% alcohol for 5 minutes, washed 5 times with sterile water: for each 10 P. vulgaris seeds were inoculated, with 1 mL of P. norvegensis at a density of 6.4 x106 CFU/mL, S. cerevisiae at a density of 8.1 x106 CFU/ml, X. autotrophicus at a density of 4.3x106 CFU/mL, then were sown in soil, according to the Table 1, under an experimental design of random blocks: six treatments and six repetitions: P. vulgaris uninoculated irrigated with water, or absolute control (AC). P. vulgaris uninoculated, fed with 100% or relative control (RC); (T1) = P. vulgaris with P. norvegensis 70% NH4NO3; (T2) =P. vulgaris with S. cervesiae fed NH4NO3 at 70%; (T3) =P. vulgaris with P. norvegensis and S. cervesiae fed NH4NO3 at 70%; (T4) = P. vulgaris with P. norvegensis and X. autotrophicus fed NH4NO3 at 70%; (T5) = P. vulgaris with S. cervesiae and X. autotrophicus with NH4NO3 at 70%; (T6)= P. vulgaris with X. autotrophicus fed NH4NO3 at 70%. The plants were fed with mineral solution with the following chemical composition (g/L): NH4Cl or NH4NO3 10.0, K2HPO4 2.5, KH2PO4 2.0, MgSO4 1.0, NaCl 0.1, CaCl2 0.1, FeSO4 traces, trace element solution 10.0 mL, adjusted to a pH of 6.5-6.8; for the trial regard as a RC so that it was 100% NH4NO3 10.0 g was applied and in the inoculated P. vulgaris to 7.0 g of NH4NO3 was added. The response variables were based on the days of emergence and percentage of germination; phenology: plant height (PH) and root length (RL); in biomass: aerial fresh weight (AFW) and radical fresh weight (RFW); for the aerial dry weight (ADW) and radical dry weight (RDW) of P. vulgaris seedling stage (Sanchez-Yañez, 2007). The experimental results obtained were analyzed by ANOVA and Tukey (P ≤ 0.05), to establish the minimum significant difference Figure 1.15
According to biochemical and molecular tests, endophytic plant yeasts were identified as P. norvegensis and S. cervesiae from Amarylis belladonna, Clivia miniata and Spanthodea campanulata respectively.
Table 1 demonstrated the positive effect of S. cervesiae and X. autotrophicus, on the germination of P. vulgaris seed with NH4NO3 at 70%, registed 97.7% germination, and the emergence was 3 days after sowing. Was evident that the seeds of P. vulgaris responded positively to all types of yeast, as well as did to X. autotrophicus, compared to seed of P. vulgaris used as a relative control, uninoculated fed with NH4NO3 at 100%, as well as with days to emergency.
Figure 2. Effect of P. norvegensis, S. cervesiae and X. autotrophicus on the germination, of Phaseolus vulgaris seeds with NH4NO3 at 70%, shows the positive effect of endophytic yeasts, and X. autotrophicus with 50% NH4NO3. There it was easy to observe, that both the stem and root primordium, showed an evident greater and better growth, compared to the seed of P. vulgaris, uninoculated with endophytic yeast and X. autotrophicus, fed with 100% NH4NO3 used as a relative control (RC), as well as the seed of P. vulgaris irrigated with water or absolute control (AC).
Table 2 shows the response of P. vulgaris in seedlings to P. norvegensis and X. autotrophicus with NH4NO3 that registered 37.48 cm of PH, 18 cm of RL, 1.96 g of AFW, 1.55 g of RFW, 0.24 g of ADW and 0.14 g of RDW, followed by the response of P. vulgaris to S. cervesiae and X. autotrophicus with NH4NO3 at 70%, that registered 36.83 cm of PH, 17.83 cm of RL, 1.93 g of AFW, 1.58 g of RFW, 0.22 g of ADW and 0.13 g of RDW. The results of the positive response of P. vulgaris in phenology and biomass to P. norvegensis and X. autotrophicus with NH4NO3 at 70% had a statistical difference with 28.8 cm of PH, 10.66 of RL, 0.82 g of AFW, 0.29 g of RFW, 0.12 g of ADW and 0.03 of RDW of P. vulgaris uninoculated by P. norvegensis or X. autotrophicus with 100% NH4NO3 or RC.
Figure 2 showed the positive response in the phenology of the P. vulgaris seedling stage to P. norvegensis, S. cervesiae and X. autotrophicus with NH4NO3 at 70 %, with an increase in the number of stems, size of leaves, intense green coloration and increase in the density of the root system, the foregoing supports the fact that P. norvegensis, S. cervesiae and X. autotrophicus improved the uptake and optimization of NH4NO3 at 70% compared to P. vulgaris uninoculated with P. norvegensis, S. cervesiae and X. autotrophicus with 100% NH4NO3 or RC that showed fewer stems and leaves together with a less dense root system, indicating that the recommended dose was not uptake and was optimized to the maximum, that cause greenhouse gasses for global warming6 acute problem and lost soil fertility that agriculture has to solve.1
Endophytic yeasts: P. norvegensis and S. cervesiae has been living with legume as P. vulgaris for long time ago, in that sense the positive interaction among them or mixing with X. autotrophicus is interesting tool for sustainable agriculture to adjust dose of nitrogen fertilizer in order to avoid greenhouse gases6 and global warming16–18 as it has showed in this research with NH4NO3 at 70% of the recommended dose for P. vulgaris where is cropping in Mexico.
In Table 1 showed these facts support when exuding the seed of P. vulgaris, released sugars such as glucose and maltose, organic acids and amino acids as sources of carbon and energy, that S. cervesiae plus X. autotrophicus transformed, into phytohormones that interrupted the latency period of the seeds, to the emergence of root and stem primordium. This corroborates the research by Tawfiq et al., 2018, whom reported that S. cervesiae was capable of synthesizing phythormones. Lencinas et al.,19 analyzed S. cerevisiae and S. pombe in Lactuca sativa seeds, reported an increase in germination per centage. The results of the positive effect of S. cervesiae and X. autotrophicus in the P. vulgaris seed, fed NH4NO3 at 70% had a statistical difference, compared to 70.7% germination and that emerged 5 days after the sowing of P. vulgaris, non-inoculated fed with 100% NH4NO3 or relative control (RC).
|
Tratamiento Phaseolus vulgaris** |
Emergency days |
Germination percent |
|
Absolute Control (AC) |
5b** |
50.6f |
|
Relative Control (RC) |
5b |
70.7d |
|
Pichia norvegensis + NH4NO3 at 70% |
3ª |
66.6e |
|
Saccharomyces cervesiae + NH4NO3 at 70% |
3ª |
94.4a |
|
P. norvegensis + S. cervesiae + NH4NO3 at 70% |
3ª |
88.8c |
|
P. norvegensis+ Xanthobacter autotrophicus + NH4NO3 at 70% |
3ª |
92.2b |
|
S. cervesiae + X. autotrophicus + NH4NO3 at 70% |
3ª |
97.7a |
|
X. autotrophicus + NH4NO3 at 70% |
3a |
93b |
Table 1 Effect of Pichia norvegensis, Saccharomyces cervesiae and Xanthobacter autotrophicus on the germination of Phaseolus vulgaris seeds with NH4NO3 at 70%
n=6, **Different letters indicate statistical difference by ANOVA Tukey P>0.05.
On Figure 2 there an increase in root primordia was observed, this supports that P. norvegensis, S. cervesiae and X. autotrophicus, accelerated plant growth by increasing root density, that improved exploration in the soil to uptake and maximize NH4NO3 at 70%. Compared to the seed of P. vulgaris uninoculated, with P. norvegensis, S. cervesiae and X. autotrophicus fed 100 % NH4NO3 or RC, there it was shown with a smaller root and stem primordium of P. vulgaris, these facts support that P. norvegensis. S. cervesiae and X. autotrophicus endophytically, in the root and stem primordium of P. vulgaris, transformed vegetal intermediate metabolites into phytohormones, inducing karyokinesis;20 increasing the number of root hairs that improved the uptake and optimization of NH4NO3 at 70%, and thus achieved healthy plant growth. In few analyzes the promoting effect of yeast plant growth has been reported, by Amprayn et al.,21 that showed the positive response of Oryza sativa, to Candida tropicalis which improved uptake of nitrogen fertilizer by O. sativa root system was improved, with to increase in the dry weight of the plant by 35%, due to synthesis of phytohormones; in that sense some investigations, reported the mechanisms of promotion of plant growth of the genus X. autotrophicus: Santos et al.,22 explain that solubilization of phosphates by X. autotrophicus, that was capable to solubilize phosphates precipitated in soil, for to uptake by P. vulgaris.23,24 When phosphates as well as unregulated NH4NO3 dose could not be uptake, by the root system of P. vulgaris efficiently, is possible to solve by applying natural microbial consortium, as we did at the present research work.22,7,2,11
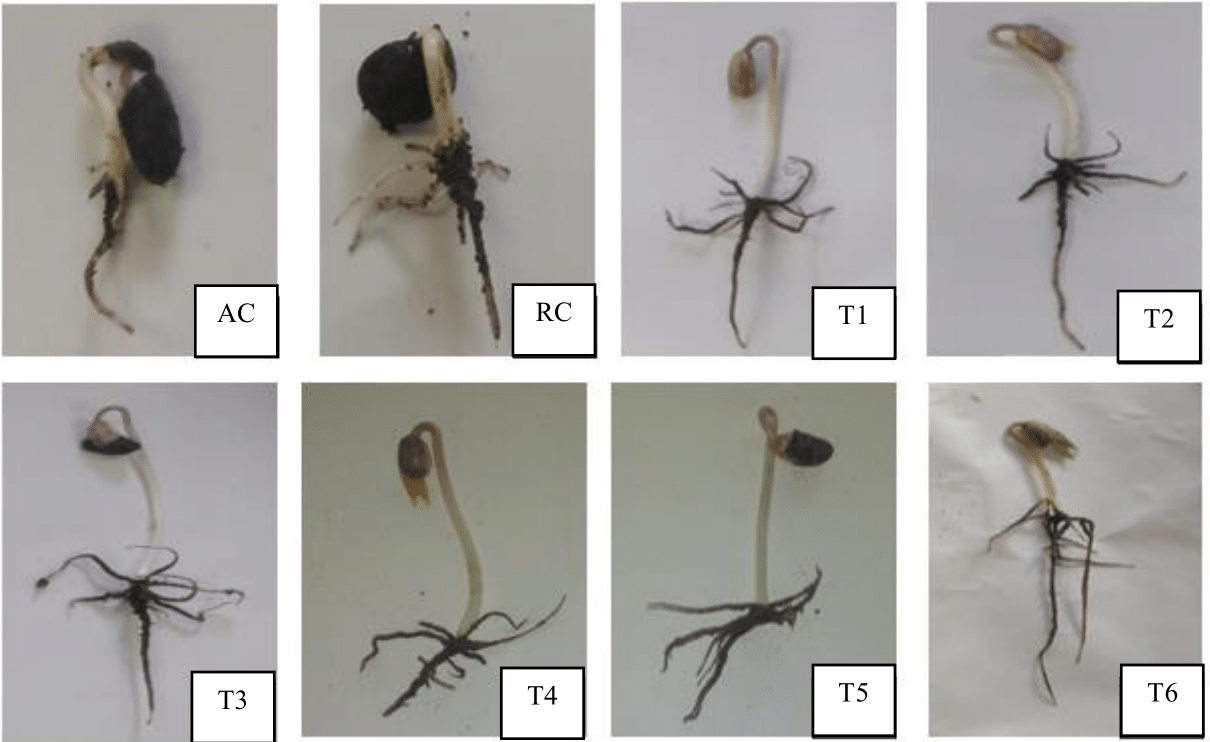
Figure 2 Effect of P. norvegensis, S. cervesiae individually or in combination with X. autotrophicus in P. vulgaris fed NH4NO3 at 70%.
AC= P. vulgaris uninoculated irrigated with water; RC= P. vulgaris uninoculated fed with NH4NO3 at 100%;
T1= P. vulgaris + P. norvegensis + NH4NO3 at 70%; T2= P. vulgaris + S. cervesiae+ NH4NO3 at 70%;
T3= P. vulgaris + P. norvegensis, + S. cervesiae + NH4NO3 at 70%;
T4= P. vulgaris + P. norvegensis + X. autotrophicus + NH4NO3 at 70%;
T5= P. vulgaris + S. cervesiae + X. autotrophicus + NH4NO3 at 70% T6= P. vulgaris + X. autotrophicus + NH4NO3 at 70%
Table 2 shows the response of P. vulgaris with 70% NH4NO3 and P. norvegensis, S. cervesiae plus X. autotrophicus. In general, regardless of which genus and species of yeast, individually or in mixture with X. autotrophicus, were able to transform organic compounds from the root metabolism of P. vulgaris into phytohormones, as an increase in root system density and the highest efficient uptake activity of NH4NO3 at 70% was observed (Agmy et al., 2013);21,10 especially in plant height (PH) and root length (RL). This showed that the endophytic yeasts and X. autotrophicus invaded the root system of P. vulgaris, so in the phenology, numerical values were recorded, statistically similar or different from the numerical values in plant height (PH) in P. vulgaris not inoculated with 100% NH4NO3.
|
Treatments * P. vulgaris |
Plant height (PH) cm |
Radical length (RL) Cm |
Aerial fresh weight (AFW) g |
Radical fresh weight (RFW) g |
Aerial dry weight (ADW) g |
Radical dry weight (RDW) g |
|
Absolute control (AC) |
21.41d** |
7.8d |
0.66d |
0.11d |
0.04c |
0.01c |
|
Relative control (RC) |
23.8d |
10.66c |
0.82c |
0.29c |
0.12b |
0.03d |
|
P. norvegensis + NH4NO3 at 70% |
30.46b |
11.46c |
1.32b |
0.37c |
0.15b |
0.03d |
|
Saccharomyces cervesiae. + NH4NO3 at 70% |
29.66b |
15.88b |
1.80ª |
0.67c |
0.18b |
0.09b |
|
S. cervesiae + P. norvegensis NH4NO3 at 70% |
34.5a |
14.76b |
1.37b |
0.63c |
0.14b |
0.07b |
|
P. norvegensis +X. autotrophicus+ NH4NO3 at 70% |
37.48a |
18.0ª |
1.96ª |
1.55ª |
0.24ª |
0.14ª |
|
S. cervesiae +X. autotrophicus + NH4NO3 at70% |
36.83a |
17.83a |
1.93a |
1.58ª |
0.22a |
0.13ª |
|
X. autotrophicus + NH4NO3 at 70% |
29.5b |
15.16b |
1.76a |
1.17a |
0.20a |
0.10ª |
Table 2 Response of Phaseolus vulgaris at seedlings stage to Pichia norvegensis, Saccharomyces cervesiae and Xanthobacter autotrophicus NH4NO3 at 70%
*n=6 **Different letters indicate statistical difference by ANOVA Tukey P>0,05.
While in P. vulgaris biomass in fresh and dry weight of aerial part (FAW/DAW) and root system with P. norvegensis, or S. cerevisiae, individually or in consortium and X. autotrophicus, a synergistic action was recorded, to transform organic compounds from P. vulgaris metabolism, into phytohormones that optimized maximum NH4NO3 uptake to 70% (Lencinos et al., 2020).4,11 Both endophytic yeasts and X. autotrophicus, share biochemical mechanisms of recognition of compounds from P. vulgaris metabolism (Tawfiq et al., 2018);15,12 dependent on NH4NO3 concentration at 70%, that facilitated a positive interaction between endophytic yeasts and X. autotrophicus with the P. vulgaris root system0.8,18 According to the numerical biomass values of P. vulgaris, endophytic yeasts in consortium with X. autotrophicus reached statistically different numerical values compared to the same parameters of non-inoculated P. vulgaris and 100% NH4NO3.
Figure 3 shows the phenology of P. vulgaris with endophytic yeasts individually or in consortium with X. autotrophicus and 70% NH4NO3 compared to non-inoculated P. vulgaris fed with 100% NH4NO3. There it was evident that in all cases the endophytic yeasts invaded the root system of P. vulgaris with or without X. autotrophicus utilized organic compounds from root metabolism to convert them into various phytohormones16,17 according to the aerial part of P. vulgaris with higher number of leaves and evident chlorophyll induction25,21,19 that was higher than in non-inoculated P. vulgaris fed with 100% NH4NO3 according to 70% NH4NO3 concentration.20,4
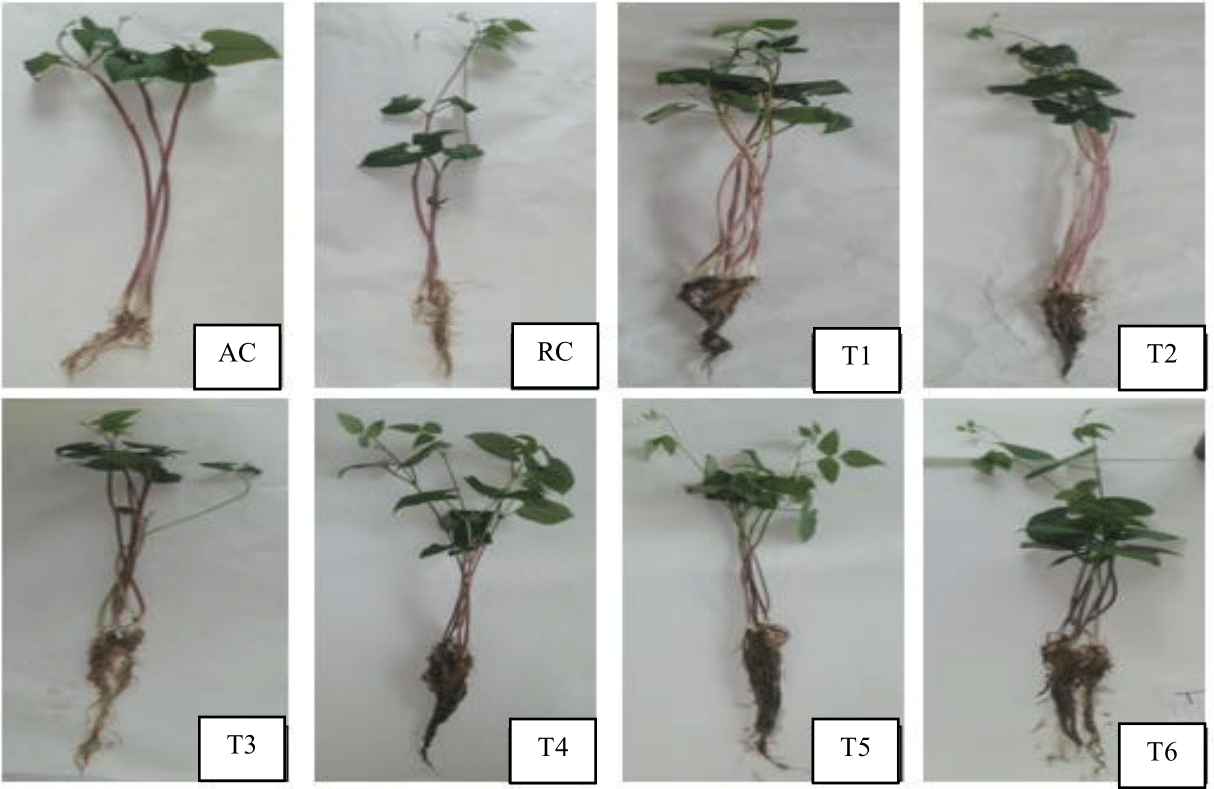
Figure 3 Response of Phaseolus vulgaris at seedlings to P. norvegensis, S. cervesiae and X. autotrophicus fed NH4NO3 at 70%.
AC= Phaseolus vulgaris uninoculated irrigated with water; RC= P. vulgaris uninoculated fed with NH4NO3 at 100%;
T1= P. vulgaris + P. norvegensis+ NH4NO3 at 70%; T2= P. vulgaris + S. cervesiae+ NH4NO3 at 70%;
T3= P. vulgaris + P. norvegensis + S. cervesiae + NH4NO3 at 70%;
T4= P. vulgaris + P. norvegensis + X. autotrophicus + NH4NO3 at 70%;
T5= P. vulgaris + S. cervesiae + X. autotrophicus + NH4NO3 at 70%
T6= P. vulgaris + X. autotrophicus + NH4NO3 at 70%
A similar positive response was observed in the dense and abundant root system of P. vulgaris treated with endophytic yeasts and X. autotrophicus due to the conversion of root metabolites into several phytohormones, so that despite reducing NH4NO3 to 70% its effective uptake without compromising the healthy growth of P. vulgaris to avoid the generation of greenhouse gases causing global warming, compared to non-inoculated P. vulgaris fed with 100% NH4NO3, that obviously the legume does not take advantage of P. vulgaris, to avoid the generation of greenhouse gases causing global warming, compared to non-inoculated P. vulgaris fed with 100% NH4NO3, that obviously the legume does not take advantage of, according to what was observed in aerial and root growth, consequently, this remnant contributes to the release of greenhouse gases.6 The growth of P. vulgaris with this natural consortium of two types of endophytic microorganisms supports the importance of the interaction between these three different types of biological groups that over time have adapted to environmental changes in the soil,26,22,15 especially the response of P. vulgaris3 dependent on the concentration of inorganic nitrogen7 as NH4NO3 available to the root system of P. vulgaris that induces some specific ecological interaction with endophytic yeasts and X. autotrophicus in harmonic association.9,4,2,27–29
Based on the above, it was demonstrated that an ancient positive interaction of endophytic yeasts from wild plants such as P. norvegensis and S. cervesiae in combination with X. autotrophicus are an alternative for the rapid and effective induction of seed germination and healthy growth of P. vulgaris at reduced doses of NH4NO3 to 70%, which facilitates the maximum uptake of nitrogen fertilizer, in turn allows a decrease in the generation of greenhouse gases, avoids the loss of soil productivity, being a useful tool for sustainable agriculture, that facilitates the maximum uptake of NH4NO3, in turn allows a decrease in the generation of greenhouse gases, avoids the loss of soil productivity, being a useful tool for sustainable agriculture. When NH4NO3 is reduced to 70%, which facilitates the maximum uptake of nitrogen fertilizer, in turn allows a decrease in the generation of greenhouse gases, avoids the loss of soil productivity, being a useful tool for sustainable agriculture.
To the Coordinación de Investigación Cientifica de la UMSNH “Aislamiento y selección de microrganismos endófitos promotores de crecimiento vegetal para la agricultura y biorecuperacion de suelos” from the Research Project 2024, Universidad Michoacana de San Nicolas de Hidalgo, Morelia, Michoacán, Mexico. For the information and experiences of the project: "Field Test of a Living Biofertilizer for Crop Growth in Mexico" from Harvard University, Cambridge, Ma, USA (2024) with support of Rockefeller fund. To Phytonutrimentos de México and BIONUTRA S, A de CV, Maravatío, Michoacán, México for the P. vulgaris seeds and verification of greenhouse tests. To Juan Luis Ignacio de la Cruz for its technical support, To Jeaneth Caicedo Rengifo for her help in the development of this research project.
The authors declare no conflicts of interest.

©2024 Sánchez-Yáñez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.