Open Access Journal of
eISSN: 2575-9086


Research Article Volume 4 Issue 2
Abdul Wali Khan University Mardan, Government Post Graduate College Nowshera KPK, Pakistan
Correspondence: Shah Hussain, Abdul Wali Khan University Mardan, Government Post Graduate College Nowshera KPK, Pakistan
Received: May 13, 2020 | Published: June 18, 2020
Citation: Hussain S. Assessment and development of carbonaceous soot from wood burning process: adsorption, kinetics and thermodynamics study for Rhodamine B dye. Open Access J Sci. 2020;4(2):52-59. DOI: 10.15406/oajs.2020.04.00152
Carbon based materials have acquired pivotal importance in the recent era for the removal of pollutants from the effluents. In this study, wooden soot (WS) obtained from wood burning has been utilized as an adsorbent for the removal of Rhodamine B dye (RB). The adsorption of RB on WS was studied as function of the contact time, concentration, and temperature. The amount of dye adsorbed onto the WS increased with the increase in the contact time, concentration and temperature. The adsorption data fitted well in Langmuir and Freundlich models with comparatively greater R2 value for the Langmuir model. Adsorption data followed the pseudo second order kinetics. The uptake of dye by the adsorbent was 238 mg/g. The various Thermodynamic parameters such as ∆Ho, ∆So and ∆Go were studied which indicated that the adsorption of RB on WS adsorbent remained a spontaneous endothermic process. The surface morphology was studied through FESEM before and after the adsorption of RB, which indicated accumulation of dye on to the small pores. The functional group analysis was performed through FT-IR which indicated a clear change at 2360 cm-1 after the adsorption which confirmed the adsorption phenomena. The pore size, pore volume and pore diameter were 47.44 m2/g, 0.07 cm3/g and 35.23 A0 respectively. This study will highly acknowledge the use of low cost adsorbent materials for the environmental remediation.
Keywords: wooden soot adsorbent, Rhodamine B dye, FESEM, Kinetics study, Thermodynamic study
Dyes constitutes a vast and diverse group of organic substances, affect our daily life directly or indirectly. Dyes industries have deeply integrated in to the human society and have grown in importance over the years. About 10,000 dyes or dyes stuff are available on the industrial level approximately 700,000 tons are manufactured each year around the world.1 Textile industries have proved itself as the back bone of the economy as it has a pivotal role in the economic development of a country especially the third world countries.2,3 The major drawback associated with these industries is the release the colored effluents into the surrounding water without any treatment as its release into water directly is much cheaper.3,4 During coloring process most of the dyes do not fix to fabric as a result a considerable amount of these unspent dyes are released into the water bodies. Literature survey has indicated that about 10–15% of the dyes stuff are released to the environment which is esthetically an un-favorable situation.5–7 The industries which release the colored effluents into the aquatic system include textile, leather, soap, dye manufacturing, paper, cosmetic and food processing. Due to their color they are the most easily recognizable pollutants.8 These dyes are the synthetically manufactured complex aromatic compounds which are highly toxic to the aquatic life as they make the water colored and thus responsible for reduction in the photosynthetic process and are damaging to the environment. Dyes are reported to cause some serious problems such as skin cancer, mutation in human beings.9–11
Due to its toxic and non-biodegradable nature they are potentially harmful to the environment .Thus the removal of these dyes from the polluted water is very essential before they are mixed with the clean water and become a potential threat for the terrestrial and aquatic biota.12–15 Environmental concerns have increased the interest in the removal of dyes from the contaminated water. The ETAD survey (Ecological and Toxicological Association of the Dye stuffs) indicated 90% of dyes among 4000 dyes were showing 2 × 103 mg/kg LD50 values. Among various class of dyes diazo and basic dyes are reported to have the highest toxicity. The UK government in Sept.1997 drafted the environmental policies for zero release of the synthetic chemical substances in the marine environment.4,6,7 Developed countries and European community are more cautious to control the dyes stuff from the industrial effluents. Even a trace amount of the dye stuff makes the water colorful which can have an obnoxious inhibiting effect on the passage of oxygen and sun light into the water body which can have a lethal effect for the aquatic flora and fauna. Therefore, it is of utmost importance for the industries to treat and purify these effluents before their release into the water resources.
Reverse osmosis, chemical coagulation, and activated sludge, are the various conventional methods used for the removal of dyes from the polluted water. Because of the stability of the modern dyes these conventional methods are not completely effective in the removal of these dyes.16 Adsorption process has been found to be an effective technology for decolonization of the waste water.17,18 For this purpose various adsorbents have been used by different researchers, for instance, industrial waste,19 agricultural products and different types of clays chitosan based and carbon based adsorbents.20–23 reported the adsorptive removal of Rhodamine B dye form aqueous solution by using acid modified banana peels.24 efficiently removed Rhodamine B dye by treating its solution with tunable organo-vermiculites.25 Found an excellent correlation of adsorption behavior of Rhodamine B dye with activated carbon derived from white sugar.24 studied an adsorbent obtained from banyan aerial roots for the adsorption of Gentian violet and Rhodamine B dyes. They found that the adsorbent effectively remove these dyes form aqueous solution at low cost. In the current research work, the main focus has been focused upon carbonaceous adsorbent derived from the wood burning process at the furnaces used for the drying of tobacco green leaves. The wood burning at these furnaces generates soot as a by-product and it necessitates its removal especially from the inner sides of the metallic pipes used in these furnaces. This wooden soot (WS) can be developed as a good adsorbent for the removal of the dyes. In this study an attempt has been made to use WS as adsorbent for the removal of Rhodamine B dye. The chemical structure of RB dye is provided in Figure 1.
The total duration of the research project remained six months from Nov 2019 to April 2020.
Reagents and Materials
All the reagents used were of analytical grade and were used without any further purification. The Rhodamine B dye was purchased from Merck. Distilled water was obtained from the Millipore Q machine at the Department of Chemistry University of Peshawar. The WS was collected from tobacco leaves drying furnace where wood burning was used for drying of green leaves of tobacco resulting in the formation of soot.
Treatment of WS
WS was treated with n-hexane in round bottom flask fitted with reflux condenser at 1:2 (mass of WS to volume of n-hexane) for overnight to washout oily components, then filtered using Whatman filter paper No. 42 and dried to remove the residual n-hexane. The dried WS sample was subsequently washed with 0.2 M NaOH solution and 0.2 M HCl for 4 h to remove ash contents. After that, WS sample was thoroughly washed with hot distilled water until pH 7.0 was achieved. The washed sample of WS was then dried in a vacuum oven at about 120 ºC until constant weight. Finally, the WS sample was grinded by using mortar and pestal and passed through 212 µm size mesh screen.
Preparation of RB dye solutions
Stock solution of 1 M concentration was prepared by mixing the required quantities of analytical grade RB dye in deionized water. Solutions with different concentrations were obtained by diluting the stock solution with distilled water.
Characterization of WS
The pore size of WS was checked through BET surface area and pore size analyzer (Model: NOVA 2200 e Quantachrome, USA). The surface morphology of the WS was examined by FESEM using scanning electron microscopy (Model: JSM-5910-JEOL JAPAN). The functional group analysis in the WS adsorbent was performed through FT-IR using FT-IR spectrophotometer (Model: IR prestige-21 Shimadzu, Japan). Powder X-ray diffraction pattern was obtained to study the amorphousness and crystalline nature of WS by using X-ray diffractrometer (Model: JDX-9C, JOEL, Japan) from 2 to 65o.
Adsorption studies
The effects of contact time, temperature and concentration of solution at constant pH of 7 and fixed ratio of mass of adsorbent to volume of solution (1: 25) on the removal of RB dye was studied using batch experiments. In each step, the desired concentration of RB dye solution was prepared by diluting stock solution with distilled water. The removal of RB dye with WS was performed in a series of experiments in different stoppered conical flasks agitated in a digitally temperature controlled shaking water bath (model SHA-C1, Thaiwan). After requisite time of shaking, the mixture was filtered with Whatman filter paper 42 and determined the concentrations of RB dye (before and after adsorption) by UV-Vis spectrophotometer (Shimadzu Japan) at the maximum wavelength of 560 nm. pH adjustment of solutions was performed with the help of bench top pH meter (HI 2210, Hanna instruments, Inc, USA). The pH meter was adjusted by using buffer solutions of pH 5.0, 7.0 and 9.0. The effect of contact time on adsorption was studied between 2 to 20 min to study maximum removal of RB dye. The effect of temperature was studied at 10 to 50oC at optimum time. The initial concentration used in batch experiments was 1 × 104- to 5 × 104-. The maximum amount of dye removed by WS was calculated by using the equation (1):
(1)
Where qe is the amount of dye adsorbed (mg/g), Ci is the initial concentration of the dye solution (mol/ dm3), while Ce is the concentration of the solution after time t, V is the volume of the dye solution used in the adsorption and W is the weight of the adsorbent used. The percent removal of RB from the solution was determined by using equation (2):
(2)
Characterization of WS
The fine size of WS was obtained after sieving through 212 mesh screen to enhance its RB dye removal capability efficiency by adsorption. The experimental result of BET analysis as provided in Table 1 reveals that WS particles were characterized by fine pore size, pore volume and larger pore diameter. The small pore size of the adsorbent suggested the high surface area of the WS. The surface morphology of the WS before and after adsorption can be seen from the field emission scanning electron microscopy (FESEM) micrographs provided in Figure 2. The variations in the SEM images before and after the adsorption of RB dyes have been pointed with red circles within the image. The result indicated that before adsorption, WS is characterized with irregular mesoporous compact structure comprising different number of fine pores as provided in Figure 2a. The pores filling after the adsorption of RB dye onto WS can be observed in SEM image as given in figure 2b which authenticate the adsorption process. Functional group and change in the structure of the adsorbent was assessed through FT-IR spectroscopy. The FT-IR spectra of the synthesized and dye loaded WS is provided in figure 3. The changes in peaks position and intensity proved RB dye adsorption (Figure 3b) on the surface of synthesized WS. The peak at 2977 cm-1 in Figure 3a is assigned to –OH group of absorb moisture. In addition, the peaks in the range of 1580 to 1390 cm−1 are correlated to stretching vibration of C=O carbonyl group of Ketones & carboxylic acid group and stretching vibration of C=C of aromatic ring.26 The peaks at 1033 and 874 cm-1 are due to C-O bending vibrations. However, the change in intensity and position of peaks in the FT-IR spectrum after RB loading confirms the adsorption process. The powdered XRD pattern of WS has been provided in Figure 3b. The XRD pattern is characterized with spiky and fine diffraction peaks in the range of 20 to 25o 2θ which correspond to carbon diffraction.

Figure 2 FESEM micrograms of the WS adsorbent: before adsorption (a) and after the RB dye absorption (b).
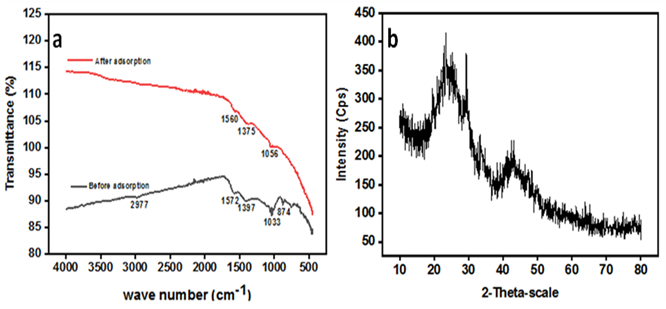
Figure 3 FTIR spectrum of WS: before adsorption & after adsorption of RB dye (a) and XRD pattern of WS (b).
pore size |
pore volume |
pore diameter |
(m2/g) |
(cm3/g) |
(Ao) |
47.44 |
0.07 |
35.23 |
Table 1 BET analysis of WS
Effects of contact time, temperature and concentration on the removal of RB
The removal of the effluents from aqueous solution is mainly dependent on their contact time with adsorbent. When contact time increases, more dye molecules get adsorbed on the active sites of the adsorbent. In the current study, the effect of time was investigated between 2 to 20 min. The removal (mg/g) of RB dye is provided in Figure 4a show that adsorption efficiency of WS. It was found that initially the rate of adsorption was very high but after 10 min, the removal of RB dye got constancy due to equilibrium establishment. The result may attribute to saturation of active site of the WS by the RB dye. The temperature effect on uptake ability of WS was experimented and provided in Figure 4b which indicated that the adsorption of the dye increases with the increase in temperature in the range of 283 to 323 K showing that the process is endothermic in nature. The increasing uptake ability of WS adsorbent may due to its nature27 because higher temperature may lead to increase or rearrange the position of the active sites.28 With the increase in concentration of the dye solution the amount adsorbed of the dye also increases. In this study 1 × 10-4 to 5 × 10-4 M solutions of RB dye was stirred with fixed amount of WS adsorbent for 10 min. The result is provided in Figure 4c which indicated that as initial concentration of RB dye increases from 1 × 10-4 to 5 × 10-4 M, more RB molecules get adsorbed by WS adsorbent.

Figure 4 Adsorption of RB dye on WS: effect of shaking contact time (a), effect of temperatures (b) and effect of different concentration of RB dye (c).
Adsorption kinetics
Order determination of the adsorption process
The kinetics of RB dye adsorption on WS was evaluated by pseudo first order and pseudo second order kinetic models based on the following expressions:29
(3)
(4)
Where qe and qt are the amount of RB dye adsorbed at equilibrium and at time t, respectively while K1 (min−1) is rate constant for pseudo first order and K2 (g mg−1 min−1) is rate constant for pseudo second order. The adsorption data of RB dye was plotted versus time to evaluate kinetic parameters both for pseudo first order and pseudo second order as provided in Figure 5a&5b, respectively. The straight lines show that the adsorption process followed both pseudo first and second order kinetics but R2 value given in Table 2 explained that pseudo second order more satisfactorily illustrated the adsorption kinetics.
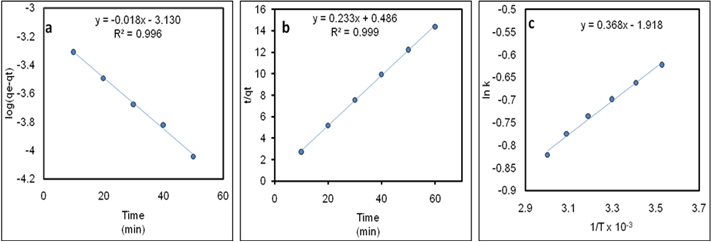
Figure 5 Kinetic study for adsorption of RB dye: plot of pseudo first order (a), plot of pseudo second order (b) and Arrhenius plot (c).
Parameters |
|||
Kinetic models |
K1 (1/ min) |
K2 (g/ mg. min) |
R2 |
pseudo first order |
0.041 |
… |
0.996 |
pseudo second order |
… |
0.537 |
0.999 |
Table 2 Various parameters for pseudo first and pseudo second order for RB dye adsorption onto WS
Determination of the activation energy
The Arrhenius equation was applied for activation energy determination.30
(4)
Where K is rate constant for pseudo second order, Ea is the activation energy, R is the gas constant and T is the absolute temperature. The activation energy value was computed from the slope of the plot of lnk versus 1/T as given in Figure 5c and the value was found to be 3.06 kJ/mole. The low value of Ea for RB dye adsorption demonstrate that the process remained physisorption.31 The Ea value as given in Table 3 shows that the process of adsorption is a physisorption rather than chemisorption.
Adsorbent sample |
Temperature (K) |
Kelvin |
Kelvin |
k value (min-1) |
ln k |
Ea |
(1/T) |
(1/T× 10-3) |
(kJ/mol) |
||||
Wooden soot (212µm) |
283 |
0.00353 |
3.53 |
0.542 |
-0.62 |
3.06 |
293 |
0.00341 |
3.41 |
0.522 |
-0.6619 |
||
303 |
0.0033 |
3.3 |
0.497 |
-0.69826 |
||
313 |
0.00319 |
3.19 |
0.479 |
-0.736 |
||
323 |
0.00309 |
3.09 |
0.46 |
-0.77523 |
||
Table 3 Various parameters for the adsorption of RB on WS adsorbent
Adsorption isotherms
Adsorption isotherms explain the interaction of adsorbate molecules with surface of an adsorbent. The adsorption data was tested by Freundlich and Langmuir models by applying Freundlich32 and Langmuir equation.33 The Freundlich equation is given in equation (5):
(5)
where x/m is the amount of adsorbate (mol) adsorbed by the adsorbent (g), Ce is the equilibrium concentration (mol/L), KF is Freundlich constant and 1/n (g/dm3) explain the adsorption capacity and intensity. A straight line was obtained by the plot of ln x/m versus ln Ce as given in Figure 6a. The values of 1/n and k were calculated from the slope and intercept of the plots of ln x/m versus ln Ce, respectively. The straight line in Figure 6a, 1/n value less than 1 and n value lying in 1–10 range proved the suitability of RB dye adsorption with high intensity.34 The Langmuir model is used to predict the favorability of adsorption under particular experimental conditions. The Langmuir equation is provided in equation (6):

Figure 6 Freundlich plot for Rhodamine B on WS (a), Langmuir plot for Rhodamine B on WS (b) and Van’t Hoff’s plot (c).
(6)
where Ce is the equilibrium concentration of adsorbate (mol/dm3), and qm is the amount adsorbed, Xm is monolayer adsorption capacity (mol/g) and KL Langmuir adsorption constant (L/mol). For the RB dye adsorption, a plot is constructed between Ce/qe against Ce which shows a straight line given in Figure 6b indicating that the adsorption process obeys the Langmuir equation very well and is favorable for RB dye adsorption on to WS. Values of Xm and KL provided in Table 4. The maximum value of Xm to remove RB dye was found 0.77 × 10-2 mol/g indicating the aggregation of greater number of molecules for the formation of monolayer. The formation of the mono saturated layer is due to the distribution of the active sites on the surface of the WS in a homogeneous way.27,35 The value of correlation coefficient (R2) as provided in Table 5 is very close to one which shows strong association of adsorption data with the Langmuir model. Further, Langmuir isotherm of RB dye on to WS was analyzed by using equation (7) in order to evaluate equilibrium parameter, RL:
(7)
W here Co is the initial concentration of RB dye.
Values of Freundlich parameter |
Values of Langmuir parameter |
||||||
KF |
1/n |
n |
qm |
Xm |
KL |
RL |
|
(1×10-2 mol/g) |
(g/dm-3) |
(mg/g) |
(1×10-2 mol/g) |
(1×10-2 L/mol) |
|||
0.11 |
0.61 |
1.61 |
238 |
0.77 |
0.98 |
0.1 |
|
Table 4 Freundlich and Langmuir parameters for the RB dye adsorption onto WS
Freundlich isotherm |
Langmuir isotherm |
|
0.988 |
0.999 |
|
Table 5 The R2 values for Langmuir and Freundlich isotherm models
The value of RL accurately predicts the adsorption process. If RL > 1, the adsorption process is favorable while it is irreversible when RL = 0. The RL and qm (mol/g) values are provided in Table 4. It shows that RL value is less than 1 which confirms that Langmuir adsorption is suitable for the RB dye adsorption onto WS. Values of Freundlich parameters shows a favorable adsorption while the maximum adsorption remained 238 mg/g according to Langmuir parameters. It is obvious from the table that the value of Xm and K1 increases with the increase in temperature which shows a favorable adsorption process.
Thermodynamic study
In order to explain the adsorption of RB dye on WS adsorbent thermodynamically, different parameters such as Gibbs free energy (ΔGo), change in enthalpy (ΔHo) and change in entropy (ΔSo) were computed by using following equations:
(7)
(8)
(9)
(10)
Where K is is the Langmuir equilibrium constant (mol/L), R is the universal gas constant and T is solution temperature (K). ln K was plotted versus 1/T as shown in Figure 6c to get straight line. The value of ∆Ho and ∆So was calculated from the slope and intercept of the plot linear variation of ln K versus 1/T, respectively.
This research was aimed to study the adsorption efficiency of WS adsorbent and to assess its uptake ability for the removal of a hazardous dye like Rhodamin B. The finding of this research indicates that WS effectively remove the selected dye from laboratory prepared aqueous solution. The BET, SEM, FT-IR and XRD analyses confirm the suitability of WS to use as adsorbent. The BET analysis indicates that larger pore diameter and volume make WS attractive for adsorption. These characteristics of WS particles leads to greater adsorption ability due to increase in interaction between adsorbent and adsorbate.36 The presence of a number of pores on WS adsorbent is also confirmed by FESEM analysis which enhances the capacity of the adsorbent to remove effluents from wastewater.37 The changes in peaks position and intensity in FT-IR spectrum after adsorption proved the adsorption of dye onto WS surface. XRD study reveals the presence of microcrystalline structure of graphite (layer by layer structure) which make it attractive for adsorption.38 The adsorption study was performed by batch method to optimized contact time of adsorbent, solution temperature and concentration of dye solution. The results reveal that 238 mg of RB dye was removed per gram of WS adsorbent. The adsorption data was fitted was kinetically studied and found that pseudo second order kinetic model whereas low Ea value illustrate that adsorption process remained physiosorption.31
Furthermore, the data was analyzed by Freundlich and Langmuir adsorption models and explained that adsorption process is suitable for RB dye removal The Freundlich and Langmuir adsorption isotherms demonstrated two different parameters. The Freundlich model enlightens the adsorption in heterogeneous systems at active sites with the formation of multiple layers. On the other hand, Langmuir model elucidates maximum removal of adsorbate and affinity for adsorption homogeneously at adsorption sites with the formation of mono saturated layer. The straight lines both for Langmuir and Freundlich isotherms reveal that adsorption process obey both models. The R2 value for Freundlich adsorption isotherm was found 0.988 while for Langmuir adsorption isotherm it remained 0.998. These results proved that the adsorption data is well fitted in Langmuir model. The values of these thermodynamic parameters are given in the Table 6. The positive value of ∆Ho shows that the adsorption process is endothermic and the positive value of ∆So shows the occurrence of some structural changes in the active sites of the adsorbent, while the negative values of the ∆Go shows that the adsorption of the RB dye on the wooden soot is physisorption and spontaneous in nature.39 The current investigation is recommends to extend the use of wooden soot in the field of adsorption as it can be used for the removal of various toxic dyes and heavy metal ions from industrial effluents.40–44
Temperature (K) |
Xm |
K1 |
ΔHo |
ΔSo |
ΔGo |
(1.10-6mol/g) |
(1.10-3L/mol) |
(kJ/mol) |
(kJ/mol. K) |
(kJ/mol) |
|
283 |
24.75 |
30.14 |
8.078 |
0.057 |
-8.069 |
293 |
25.12 |
36.72 |
-8.639 |
||
303 |
27.85 |
38.3 |
-9.21 |
||
313 |
28.81 |
43.26 |
-9.78 |
||
323 |
31.34 |
46.98 |
-10.351 |
Table 6 Values of the monolayer capacity (Xm) and binding energy constant (K1) and various thermodynamic factors for Rhodamine B adsorption on wooden soot (particle size 212µm) at different temperatures
Temperature |
Thermodynamic parameters |
||
(K) |
ΔG° (kJ/mol) |
ΔH° (kJ/mol) |
ΔS° (J/mol.K) |
283 |
-11.97 |
14.03537 |
88.76347 |
-12.86 |
|||
293 |
|||
-13.74 |
|||
303 |
|||
-14.63 |
|||
313 |
|||
Table 7 Thermodynamic parameters for the RB dye adsorption onto WS at different temperatures
In this study the carbonaceous WS material was obtained from the wood burning process are applied as an effective and low cost adsorbent for the removal of RB dye from the effluents. The WS proved itself as an efficient adsorbent to remove RB dye from aqueous solution. Maximum removal was observed at 333 K after 10 min of stirring. The removal ability of the RB dye by WS was appreciable with 238 mg/g. The adsorption data well fitted in both Langmuir and Freundlich adsorption isotherms. However, the Langmuir model with R2 value 0.999, comparatively remained better. Various thermodynamic parameters were assessed which show that adsorption of RB dye is endothermic and spontaneous in nature. It can concluded that the WS can be used as good adsorbent for RB dye and hence can be used for the removal of other hazardous textile dyes from effluents. Dyes are produced annually in tones amount most of these dyes are released in the environment while little of it is used which is alarmingly making the environment potentially harmful as most of them are carcinogenic, mutagenic and teratogenic. The discovery and use of the low cost efficient adsorbents like the wooden soot (WS) is no less than a mile stone in curbing the serious environmental problem or at least reducing its hazardous effect.
None.
All the authors confirmed that the content of this manuscript has no conflict of interest.
This research didn’t receive any fund or grant from any public or private funding agency.

©2020 Hussain. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.