eISSN: 2574-9927


Research Article Volume 3 Issue 5
Department of Inorganic and Organic Chemistry, Universitat Jaume I, Castellón, Spain
Correspondence: Barrachina E, Department of Inorganic and Organic Chemistry, Universitat Jaume I, Castellón, Spain
Received: September 25, 2019 | Published: October 31, 2019
Citation: Lyubenova ST, Fraga D, Barrachina E, et al. Vitrification and sinter-crystallization of fly ash with glass cullet. Material Sci & Eng. 2019;3(5):189-193. DOI: 10.15406/mseij.2019.03.00112
The synthesis of a new glass-ceramic obtained by sinter-crystallization has been investigated by using soda-lime-silicate glass waste and fly ashes from a coal power thermal station located in Andorra (Teruel, Spain). An original glass as frit with composition of 50wt% recycled soda-lime glass, 25wt% bottom ash, 15wt% fly ash and 10wt% CaCO3 has been melted. After sinter-crystallization at 850ºC, it has precipitated two main crystalline phases: sodium anorthite and the hedenbergite. The linear shrinkage is 1/3 of the value of conventional porcelainized stoneware and the water absorption of this glass-ceramic is similar to a conventional porcelainized stoneware tile (less than 1wt%), being the apparent density slightly higher than this type of tiles (2.6g/cm3 instead of 2.4g/cm3). Flexural strength is near twice than porcelainized stoneware (around 950kg/cm2 instead of 550kg/cm2) (95MPa in the new glass- ceramic with respect to 55MPa for the above mentioned as reference material).
Keywords: recycling, industrial wastes, glass-ceramics, sinter-crystallization; fly ashes
Nowadays, in this second decade of this century, the industrial residues they continue generating environmental problems. Such of these wastes (glass cullet and bottom or fly ashes from the coal power stations) are still abundant and not definitive applications or solutions for its immobilizing have been given, though a considerable amount of research has been conducted in the last years. One of the promising applications proposed by laboratories research has been their potential for being used in the construction industry.1,2 With respect the glass cullet from the conventional glass there is abundant research carried out in the last decades of 20th and beginning of this 21st century.3–5 Vitrification process has been demonstrated is an adequate processing method for inertize toxic and abundant residues and even to facilitate their recycling as secondary raw materials in ceramics and glasses industries.6 Transforming of starting glasses after vitrification into glass-ceramics by controlled thermal treatment is possible to reach immobilizing of a wide range of industrial wastes (mineral residues, sludges from dumps, slags, ashes,). Besides, the low cost and great availability of waste make these glass-ceramics materials very attractive from an economical and technological point of view, so synthetic high-performance materials with broad applications in construction and civil engineering can be obtained from residues.7 Therefore, it has been the aim of this research to explore the synthesis of a new type of glass-ceramic by the sinter-crystallization process from soda-lime-silicate glass and several ashes from a coal power thermal station located in Andorra (Teruel, Spain).
The batch original composition for melting of an “mother or original” glass able for being transformed in a glass-ceramic was: 25 wt% bottom ash (from Andorra) , 15wt% fly ash (from Andorra), 10wt% calcium carbonate (industrial CaCO3) and 50wt% of glass cullet (from the recycling glass sector). The chemical composition determined by XRF analysis of wastes and raw materials is shown in Table 1. X-ray fluorescence (XRF) dispersion wavelength equipment was the model S4 Pioneer – Bruker. The same Table 1 includes the XRF analysis of the final glass-ceramic. This mixture has been melted at 1500ºC during 1 hour in a lift furnace by using a refractory crucible and then, the glass composition has been quenched in cold water to obtain glassy granules, ready to be wetly milled in an alumina ball mill under 45μm. The dried glassy powder (24h at 110ºC) has been moistened at about 10 wt% to be pressed with a Nannetti® uniaxial press up to a pressure of 30MPa in the shape of rectangular glassy pieces. The pieces were subjected to the thermal firing cycle in a muffle kiln (Nannetti®), which has been achieved by varying the maximum temperature (700ºC-1000ºC) and the residence period (30min/10h) in order to elaborate the corresponding Time-Temperature-Transformation diagram (TTT). The different heat treated samples at successive temperatures and time for drawing the TTT diagram was characterized by XRD and SEM/EDS. Crystalline phases were identified X-ray diffraction (XRD) and by using a Bruker-AXS D4 Endeavor equipment (using Ni-filtered Cu-Kα radiation with scanning speed of 2º (2) per minute and registering the diffraction pattern in the 10º-80º Bragg angle range. The microstructure was observed by scanning electron microscopy (SEM) JEOL 7001F with energy dispersive X-ray spectrometer (EDS) operating in the 15-20kV interval.
wt% |
Na2O |
MgO |
Al2O3 |
SiO2 |
P2O5 |
K2O |
CaO |
TiO2 |
Fe2O3 |
LOI* |
||||||||
|
|
|
Wastes |
|
|
|
||||||||||||
Glass |
12,59 |
3,75 |
0,85 |
73,16 |
0,01 |
0,30 |
8,94 |
0,05 |
0,10 |
0.10 |
||||||||
Slag |
- |
1,13 |
23,85 |
43,19 |
0,31 |
1,13 |
5,22 |
0,72 |
23,85 |
0,20 |
||||||||
Fly ash |
0,20 |
1,23 |
26,63 |
44,44 |
0,41 |
1,23 |
5,53 |
0,92 |
18,43 |
0,15 |
||||||||
CaCO3 |
- |
- |
- |
- |
- |
- |
55,98 |
- |
- |
44,01 |
||||||||
|
|
Glass-ceramic |
|
|
||||||||||||||
Glass- ceramic |
6,83 |
2,10 |
17,05 |
52,75 |
- |
0,78 |
12,34 |
0,36 |
7,69 |
0,03 |
||||||||
Table 1 Semiquantitative analysis (XRF) of industrial wastes and the glass-ceramic
*LOI, loss on ignition
The physical and technological properties, such as apparent density (hydrostatic balance method), flexural strength (determined by a HOYTOM® plasticinometer under load cell of 5000 N and 16N initial force), linear shrinkage (Mitutoyo® digital caliper) were determined in the original glass and glass-ceramics. Linear shrinkage was determined, as is usual by considering the initial length (Li), the length of the dried piece and the final length (Lf) of the heat treated sample: %Sh=(Li-Lf)/Li•100. Water absorption has been determined according to the Quality Normative for Ceramic Tiles (UNE-EN-ISO 10545-3).
The final composition of original glass and corresponding glass-ceramic was: SiO2 (52.75wt%), Al2O3 (17.05wt%), CaO (12.34wt%), Fe2O3 (7.69wt%) and Na2O (6.83wt%).
Experimental time-temperature-transformation diagram (TTT diagram)
The experimental TTT diagram which has been represented in the Figure1 shows the “nose curve” dividing the three main zones: a) the amorphous area outside the curve, where the glass stay vitreous without depicting diffraction peaks; b) the zone inside the nose curve where the crystallites nucleate and growth and c) the superior outside nose curve, where takes place the softening of glass and melting. Besides, X-ray diffractograms corresponding to samples fired for 30min are displayed in Figure1B.

Figure 1 A) TTT Diagram of the composition. B) X-ray diffractograms from samples treated during 30 minutes at successive temperatures from 800 to 1000ºC.
Microstructural morphology
The microstructural morphology of heat treated samples during 30 minutes has been observed by SEM (Figure 2).
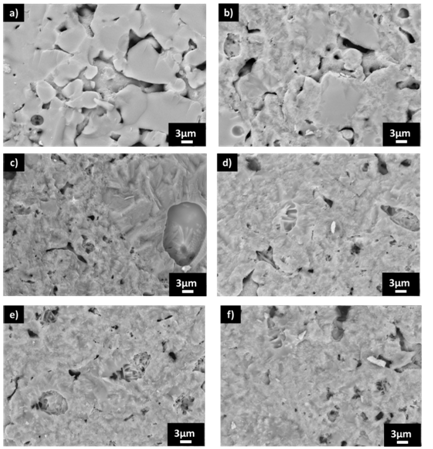
Figure 2 Microstructure observed by SEM from different samples heat treated during 30 minutes at A) 800ºC, B) 850ºC, C) 900ºC, D) 950ºC, E) 975ºC, F) 1000ºC.
Ceramic properties
The variations on apparent density and flexural strength vs temperature are displayed in Figure 3a, while the linear shrinkage and water absorption vs temperature are plotted in Figure 3B. These parameters are essentials to control the quality of the final product, because it has a ceramic application, being susceptible to cover floors and walls.
About the chemical composition, it is expected the devitrification of crystalline phases belonging to the more common simplified CaO-Al2O3-SiO2 ternary system, in which the fly ashes are usually located and mineral substitutions due to transition element oxides.1 Referring to the TTT diagram, the crystallization occurs from 850ºC and is independent of time of heat treatment (Figure 1A). Besides, 850ºC is a relatively low temperature that together with the short time of operation, become an economically profitable industrial process. The X-ray diffractograms of samples treated at 800-1000ºC during 30 minutes of residence time are shown in Figure 1B. The sample tested at 800°C is completely amorphous, whereas the samples subjected to temperatures from 850ºC to 950ºC show the most intense peaks of the solid solution of sodium anorthite (JCPDS card No. 86-1650), a mineral phase of anorthite where some CaO has been substituted by Na2O, which is present in the glass-ceramic (Table 1) in an approximate content of 7wt% and hedenbergite (JCPDS card No. 70-1876), a diopside-pyroxenic phase with iron substitutions. While this sodium-anorthite presents its maximum intensity at 900ºC, the hedenbergite shows the maximum crystallinity at 950ºC. Both phases exhibit competitive mechanical properties.
About microstructural morphology, at 800ºC, the corresponding SEM micrograph shows the presence of glassy particles partly sintered and without signs of crystallization, while at 850ºC the vitreous particles show heterogeneities of crystals nuclei. Since 900ºC to 1000ºC micrographs exhibit the presence of crystals inside the sintered particles. The average chemical analysis by EDX of the crystals in these crystallized samples shows the following oxides: 6.0 wt% Na2O, 0.9 wt% MgO, 15.5 wt% Al2O3, 61.2 wt% SiO2, 0.8 wt% K2O, 8.8 wt%, CaO, 6.7 wt% Fe2O3, showing that there is a mixture of both phases, the sodium anorthite, and the hedenbergite. Figure 3A shows density values with a maximum value (2.60 g/cm3) is reached 850ºC, been more or less constant until 1000ºC, in samples heat treated at 30 minutes. This value is higher and is obtained at lower temperatures than in the case of porcelain ceramic tiles, which reaches an only apparent bulk density of 2.40g/cm3 at 1200ºC. The flexural strength increases with temperature, achieving the maximum (around 95MPa) at 950ºC when the hedenbergite shows the higher value. At higher thermal treatment temperature (1200º C), the resulting tile shows a conventional value of 60MPa. The linear shrinkage (Figure 3B) shows the same behavior than the apparent density and reaching the maximum (around 2.5%) at 850ºC, remaining almost constant until 1000ºC. In contrast, the water absorption (Figure 3B) exhibits a large reduction at 850ºC, where the maximum linear shrinkage is obtained, holding at similar values of 1% until 975ºC. After 975ºC continues its reduction from 1% to 0.5 %, because the vitreous phase is growing whilst crystals are dissolving, a phenomenon which gives rise to the porous sealing, as can be seen in the SEM micrographs (Figure 2F). Compared to a conventional porcelainized stoneware tile, this glass-ceramic material exhibits a much lower shrinkage (2.5% with respect the 7.5% of porcelain tile) and similar water absorption at maximum apparent density (in both materials less 1%). Therefore, a conclusion it is evident the advantages of using glass cullet for obtaining a new type of glass-ceramic tiles.
It has been obtained a high-resistance glass-ceramic tile formulated from industrial residues (50% glass cullet, 25% bottom ash, 15% fly ash and 10% CaCO3), which exhibits better technological properties (2.60g/cm3 apparent density and 95MPa flexural strength) than conventional porcelainized stoneware tiles.
This research was funded by the Spanish Government through the National Research Programs: RETO INVESTIGACIÓN “SUNBEAM” (ENE2013-49136-C4-2-R) and RETO COLABORACIÓN “ECOART” (RTC-2014-2294-3).
The authors thank J. Ma. Rincón for reviewing and correcting this manuscript.
The authors declare no conflict of interest.

©2019 Lyubenova, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.