eISSN: 2574-9927


Research Article Volume 6 Issue 3
Department of Chemistry, Morgan State University, USA
Correspondence: Maurice O. Iwunze, Department of Chemistry, Morgan State University, USA, Baltimore, MD 21251, Tel +1 443- 885-3634, Fax +1 443- 855- 8286
Received: August 09, 2022 | Published: August 15, 2022
Citation: Iwunze MO. The fluorescence study of the quenching of nanoemulsion by protoporphyrin IX (PPIX). Material Sci & Eng. 2022;6(3):97-99 DOI: 10.15406/mseij.2022.06.00185
Nanoemulsion is a unique and versatile fluid that solubilizes both ionic and non-ionic molecules. It has been used extensively for drug delivery. Nanoemulsion, prepared with water-oil-surfactant and co-surfactant, is fluorescent at or the near IR region of electromagnetic spectrum. However, when protoporphyrin (PPIX), a useful photosensitizer in PDT modality, and a poorly water-soluble compound, is introduced into the nanoemulsion medium, the observed fluorescence of the nanoemulsion is quenched. We have used the steady-state fluorescence technique to study the mechanism of this quenching. It is observed that the interaction between nanoemulsion and PPIX is 1:1 and the interaction constant, Ka, is about 2.37 x 105/mol and the Free energy of interactions, Ga, is -30.66 kJ/mol.
Keywords: nanoemulsion, fluorescence, porphyrinix, complexation, binding constant, bimolecular quenching constant
CTAB, cetyltrimethyl ammonium bromide; ∆Ga, free energy of association; Ka, association constant; KSV, stern-volmer constant; kq, bimolecular quenching constant; NEM, nanoemulsion; PDT, Photodynamic therapy; PPIX, protoporphyrin IX; o/w, oil-in-water
Nanoemulsion is a heterogeneous fluid system that is extensively used for drug delivery.1-7 It is also used to solubilize both ionic and non-ionic molecules. It is also found useful in food industry8 It is a colloidal system made by dispersing oil in water with a dispersing agent, surfactant, and co surfactant, usually a short chain alcohol, in appropriate ratios. The dispersing agent used in this work is cetyltrimethylammonium bromide (CTAB). The composition of the nanoemulsion used in this work is listed in Table 1 Nano emulsion fluoresces intensely at or near the near-IR region3-9 of the electromagnetic spectrum – a region that is most useful for biological analysis. On the other hand, protoporphyrin, PPIX, is a poorly water-soluble10-15 photosensitizer but it is quite soluble in nanoemulsion. However, when PPIX is mixed with nanoemulsion the fluorescence intensity of the nanoemulsion is decreased. To the best knowledge of the author, there has not been any study in the association or complexation of nanoemulsion with PPIX. We have, therefore, used the steady-state fluorescence technique to study the observed mechanism of this quenching. Figure 1 shows the SEM image of the prepared nanoemulsion and the structure of PPIX.
Chemicals
The entire chemical used (tetradecane (Oil), surfactant (CTAB), 1-pentanol) were of analytical reagent standard and were obtained from Acros Chemicals and used without further purification.
Instrument
The Fluorescence spectra were obtained using Perkin Elmer’s Luminescence Spectrophotometer, model LS 50 B. All solutions were prepared using triply distilled deionized water from Photronix Reagent Grade water system.
All fluorescence data were obtained in a four-sided cuvette. The excitation wavelength was at 400 nm and the emission was observed at 795 nm. The instrument slit width (excitation and emission) were kept constant at 3.5 nm.
Preparation of nanoemulsion
The preparation of the nanoemulsion used in this work followed the literature methodology.14 Briefly, a measured weight of 12.0 g of CTAB as added to 174 mL of water and the mixture formed a slurry. This slurry was mechanically stirred for about two or three minutes after which 18.25 mL of n-tetradecane (oil) were added to the slurry dropwise while the mixture is still being stirred. Thereafter 31.80 mL of n-pentanol were added, again dropwise. The stirring continued until the mixture became clear and translucent. The translucent solution was transferred to an ultrasonic sonicator where it was sonicated for about 10 minutes. The nanoemulsion so prepared was found to be isotonic, clear, and translucent and was found to be stable for a considerable length of time. The nanoemulsion so prepared may be said to be oil in water (o/w) nanoemulsion. The chemical compositions used for preparation of the used nanoemulsion are shown in Table 1.
Component |
Wt., g |
Percentage, % |
Volume, mL |
Water |
174 |
76 |
174 |
CTAB (Surfactant) |
12 |
5 |
12.63 |
Oil (Tetradecane) |
14 |
6 |
18.25 |
Co-Surfactant (1-pentanol) |
29.9 |
13 |
31.8 |
Table 1 Composition of the prepared nanoemulsion
Methodology
All solutions were made in the prepared nanoemulsion. Different aliquots were used in dissolving and diluting different amounts of PPIX in 5.0 mL volumetric flasks. Ten flasks were used and the first flask contained no PPIX and the rest contained PPIX concentration that varied from 6.45 x 10-7 M to 3.24 x 10-6 M PPIX.
The fluorescence measurements were made by adding 3.0 mL of each solution to a 3.5-mL of a four-clear sided cuvette. The excitation was at 350 nm and the emission was observed at 695 nm. The instrument slit widths (excitation and emission) were kept constant at 5 nm.
We show in Figure 2 the fluorescence spectra of nanoemulsion with different concentration of PPIX As can be seen in this figure, the fluorescence intensity of nanoemulsion decreases with an increase in PPIX concentration. This observed phenomenon is consistent with molecular quenching experiments. We show in Figure 3 the ratio of the observed fluorescence intensity without, Io and with, I, of the PPIX concentration in accordance with the stern-Vollmer equation:
(1)
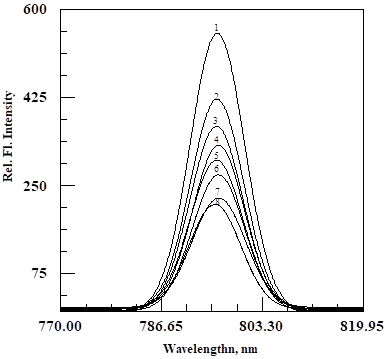
Figure 2 The Fluorescence spectra of Nanoemulsion with and without PPIX.
1= 0 (see methodology in text), = 6.45x10-7 , 3 = 9.073 x 10-7, 4. = 1.1664 x 10-6, 5. = 1.296 x 10-6, 6. = 1.944 x 10-6, 7. = 2.8572 x 10-6, 8. = 3.24 x 10-6
This plot is quite linear with a correlation coefficient of 0.998, implying that the observed data obeys the Stern-Volmer equation very well. In this equation, kq and τ are the bimolecular quenching constant and the fluorescence lifetime of nanoemulsion without a quencher, respectively. KSV and Q are, respectively, the Stern-Volmer constant the quencher concentration, where Q is the PPIX in this study.
From the relation in equation 1 above, KSV = kqτo . We have approximated the lifetime, τo, of nanoemulsion to be 4.0 ns16-18 to obtain kq value of 1.22 x 1014 M-1s-1. This value is about five orders of magnitude larger than the kq in diffusion-limited quenching of most biomolecules obtained in water which is about 2.0 x 10-10 M-1s-110-21. We then assume that the observed quenching of nanoemulsion by PPIX is by collision and subsequent ground-state complex formation. For this reason, use is made of the equation developed by Bai and his co-works equation given in equation 224. This equation has been used successfully by other workers19,22-24 to further analyze and obtain some relevant data for this quenching experiment.
(2)
In this equation, K and Q are the binding constant and quencher, respectively, n is the binding or complexation ratio. I and Io are the fluorescence intensity with and without quencher. When the obtained fluorescence data were plotted according to this equation, a linear curve is obtained as can be seen in Figure 4.
From intercept and slope of the plot, he values of K and n were determined as 2.37 x 105/mol and 0.941 ≈ 1, respectively. The free energy of interaction, ΔGa, was calculated using the relation:
ΔG = -RTlnK. A value of -30. kJ/mol
We list in Table 2, the relevant values of the quenching reaction of nanoemulsion by PPIX.
|
Parameter |
Value |
Unit |
|
KSV (Stern-Volmer Quenching constant) |
4.89 x 105 |
M-1 |
|
Kq (bimolecular quenching rate constant) |
1.22 x 1014 |
M-1s-1 |
|
K (Binding Constant) |
2.37 x 105 |
mole-1 |
|
n |
1 |
|
|
∆G |
-30.66 |
kJ/mole |
Table 2 Parametric values obtained in the quenching of Nanoemulsion by PPIX
We have shown in this work that nanoemulsion is fluorescent and the fluorescence intensity is quenched by PPIX. The observed quenching is static, collisional and complexation with a binding ration of 1:1. The interaction constant was determined as 2.37 x10.5/mol. This interaction is very exergonic and spontaneous with a free energy of --30.66 kJ/mol.
The author is very grateful to the Chemistry department of Morgan State University for her support of this work.
The author hereby declares of having not conflict of interest in this article.
None.

©2022 Iwunze. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.